Kit for real-time fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection of poliomyelitis virus
An RT-PCR and polio technology, which is applied in the field of real-time fluorescent RT-PCR detection kits for poliovirus, to achieve the effects of improving reliability and accuracy, and avoiding false negatives and false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Development of poliovirus detection reagent
[0035] 1. Design of primers and probes: by comparing and analyzing the nucleic acid sequences of reported polioviruses, the poliovirus pol gene was used as the amplification target site, and a highly conserved region with no secondary structure was selected According to the basic principles of primer-probe design, multiple pairs of primers and probes are designed by software and manually.
[0036] 2. Selection of clinical samples: According to relevant domestic and foreign literature reports, samples such as feces, cerebrospinal fluid, and serum can be used
[0037] 3. Establishment and optimization of the reaction system
[0038] Sample preparation: use the constructed pseudovirus containing the target amplification region as a positive quality control for poliovirus detection; use enterovirus EV71, Coxsackie virus A16, Japanese encephalitis virus, influenza virus type A, Influenza virus type B and Echo viru...
Embodiment 2
[0047] Example 2: Poliovirus detection kit and its use
[0048]1. Prepare a kit including the following components: 1 tube of RNA extraction solution (50ml / tube), 1 tube of polio primer-probe mixture (50μl / tube), 1 tube of RT-PCR reaction solution (720μl / tube) 1 tube of RT-PCR enzyme system (150 μl / tube), 1 tube of negative quality control product (200 μl / tube), 1 tube of positive quality control product of polio.
[0049] 2. Specimen collection, transportation and storage
[0050] Specimens will be collected by clinicians according to the actual situation. Testable specimens include throat swabs, nasopharyngeal secretions, and serum. The collection methods are as follows: ① Fecal samples: Take an appropriate amount of specimens to prepare sample suspension according to the proportion, centrifuge to get the supernatant, keep it sealed, and send it for inspection at low temperature; ② Cerebrospinal fluid: The collection time is within 3 days after the onset of neurological sym...
Embodiment 3
[0065] Example 3: Application of poliovirus detection kit to detect samples
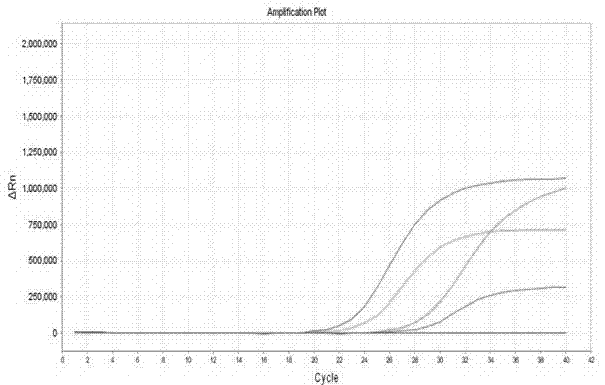
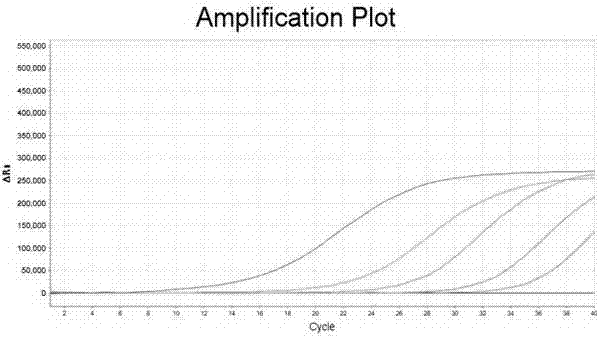
[0066] With positive quality control substance, negative quality control substance, specific reference substance in embodiment 1 as samples to be tested, detect these samples respectively according to the method of embodiment 2, positive quality control substance detection result is positive, negative quality control substance and Specific reference test results were all negative (see figure 1 ,2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com