Andrographolidume sustained-release preparation as well as preparation method and use of same
A technology of total andrographolide and sustained-release preparations, which is applied in the field of sustained-release preparations of total andrographolide, which can solve the problems of inability to obtain mechanical parameters of sustained-release preparations of traditional Chinese medicine, increased formulation design of sustained-release excipients, and difficulty in guiding the design of sustained-release preparations of traditional Chinese medicine and other issues, to achieve the effect of improving bioavailability, reducing medical burden, and uniform drug release speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 total andrographolide sustained-release preparation of the present invention
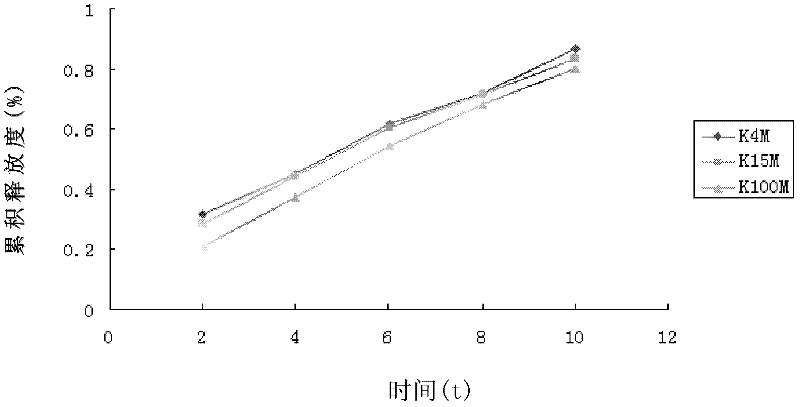
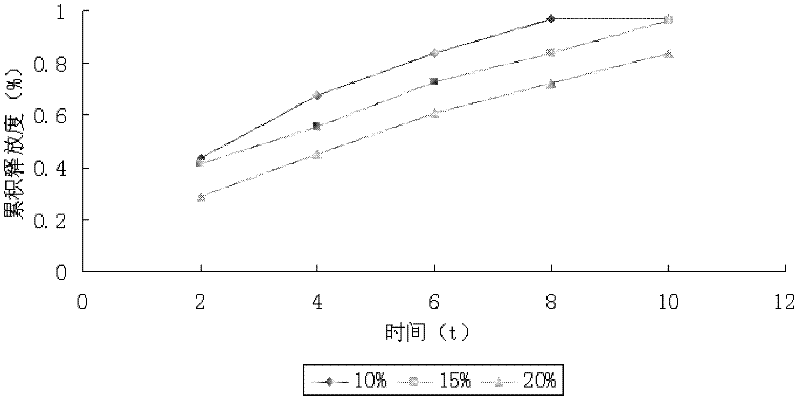
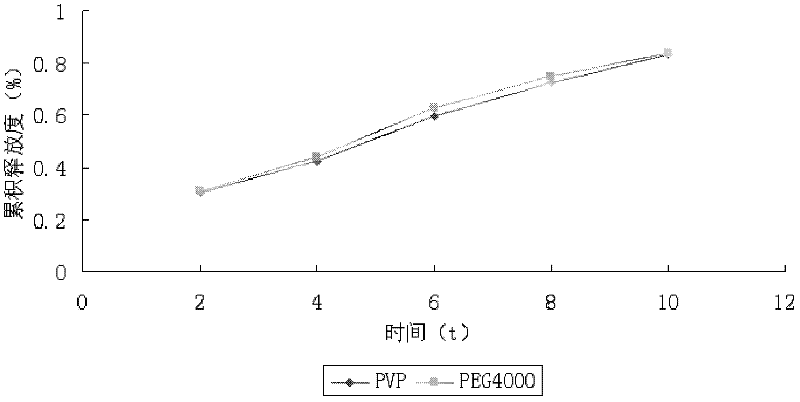
[0033] Weigh andrographolide 80g, HPMC K15M 60g, lactose 111g, starch 37g, PVP9g, andrographolide, HPMC K15M, lactose, starch, PVP were passed through 80 mesh sieve respectively, and andrographolide and auxiliary materials were pressed Mix evenly by incremental method, add appropriate amount of binder to make soft material, make granules, dry at 60°C for 1 hour, sieve No. 2 sieve, add 1% lubricant magnesium stearate based on the weight of the granules, and control the pressure at 7-9kg .cm -1 Instantly. Each tablet of this product contains about 80mg of total andrographolide, and the weight of the tablet is about 300mg.
[0034] Wherein, the total andrographolide used is the extract of the dry aerial part of Andrographis paniculata (Burm.f.) Nees, a plant of the genus Andrographis of the family Acanthaceae, and the total andrographolide contained in the extra...
Embodiment 2
[0035] The preparation of embodiment 2 total andrographolide slow-release preparations of the present invention
[0036] Get total andrographolide 78g, HPMC K15M 60g, lactose 112.5g, starch 37.5g, PVP9g, after total andrographolide, HPMC K15M, lactose, starch, PVP are passed through 80 mesh sieves respectively, total andrographolide and auxiliary material are pressed Mix evenly by equal amount incremental method, add an appropriate amount of binder to make soft material, make granules, dry at 60°C for 1 hour, sieve No. 2 sieve, add 1% lubricant magnesium stearate based on the weight of the granules, and control the pressure at 7- 9kg.cm -1 Instantly. Each tablet of this product contains about 80mg of total andrographolide, and the weight of the tablet is about 300mg.
[0037] Wherein, the total andrographolide refers to the total lactone extract prepared from the Andrographis paniculata (Burm.f.) Nees, a plant of the genus Andrographis paniculata (Burm. Andrographolin, not ...
Embodiment 3
[0038] The preparation of embodiment 3 total andrographolide sustained-release preparations of the present invention
[0039] Get total andrographolide 81g, HPMC K15M 60g, lactose 110.25g, starch 36.75g, PVP9g, after total andrographolide, HPMC K15M, lactose, starch, PVP are passed through 80 mesh sieves respectively, total andrographolide and auxiliary material are pressed Mix evenly by equal amount incremental method, add an appropriate amount of binder to make soft material, make granules, dry at 60°C for 1 hour, sieve No. 2 sieve, add 1% lubricant magnesium stearate based on the weight of the granules, and control the pressure at 7- 9kg.cm-1 Instantly. Each tablet of this product contains about 80mg of total andrographolide, and the weight of the tablet is about 300mg.
[0040] Wherein, the total andrographolide used is extracted according to the method described in CN200910115426.0.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com