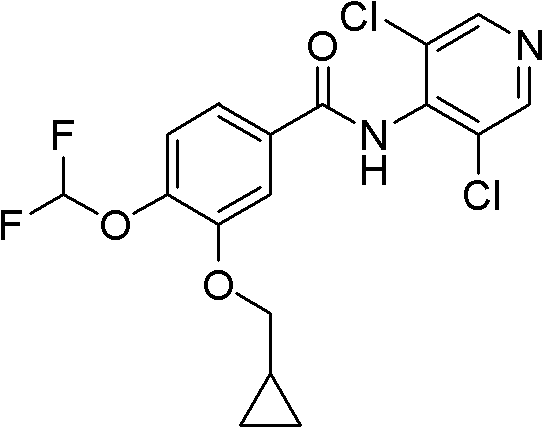
Method for preparing Roflumilast
A technology of roflumilast and difluoromethoxybenzaldehyde is applied in the field of preparing N--3-cyclopropylmethoxy-4-difluoromethoxybenzamide, which can solve the problem of low yield and difficult Purification, many by-products and other problems, to achieve the effect of high reaction selectivity, simple post-treatment, easy to obtain raw materials and
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 3-cyclopropylmethoxy-4-methoxybenzaldehyde formula (2):
[0031] 1.3kg isovanillin (8.55mol), 1.768kg (13.09mol) bromomethylcyclopropane, 1.768kg (12.8mol) anhydrous potassium carbonate, 6.175L anhydrous dimethylformamide, 6.5 g benzyltriethylammonium chloride (0.028mol), stirred at 30°C. The system is yellow and turbid, and gradually turns white as the reaction progresses. After reacting for 9 hours, TLC detected that the reaction was complete.
[0032] After the reaction was completed, filter, the filtrate was concentrated under reduced pressure, dissolved in 5L ethyl acetate, washed five times with 1L saturated sodium carbonate solution, washed with water until neutral, dried over sodium sulfate, concentrated under reduced pressure to obtain 1.655kg (8.03mol) of off-white solid, yield 93.8% (HPLC purity 99%)
Embodiment 2
[0034] Synthesis of 3-cyclopropylmethoxy-4-methoxybenzaldehyde formula (2):
[0035] 1.3kg isovanillin (8.55mol), 1.185kg (13.09mol) chloromethylcyclopropane, 1.768kg (12.8mol) anhydrous potassium carbonate, 6.175L anhydrous dimethylformamide, 6.5 g benzyltriethylammonium chloride (0.028mol), stirred at 80°C. The system is yellow and turbid, and gradually turns white as the reaction progresses. After reacting for 9 hours, TLC detected that there was still a small amount of unreacted raw material in the reaction.
[0036] Filtrate, concentrate the filtrate under reduced pressure, dissolve in 5L ethyl acetate, wash with 1L saturated sodium carbonate solution five times, wash with water until neutral, dry over sodium sulfate, concentrate under reduced pressure to obtain off-white solid 1.12kg (8.03mol), yield 93.5% ( HPLC purity 96%)
Embodiment 3
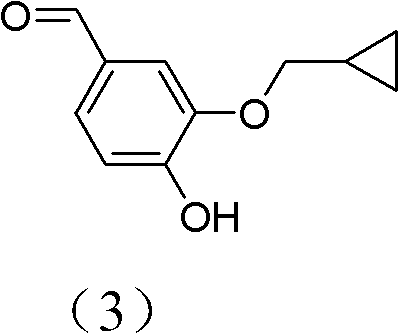
[0038] Synthesis of important intermediate formula (3) of roflumilast:
[0039] Add 2.68kg dodecanethiol (13.24mol), 716.8g (13.27mol) sodium methylate, 6kg dimethylformamide, 12g benzyltriethylammonium chloride (0.052mol) successively in the 20L reaction flask, 1.6kg ( 7.77mol) of 3-cyclopropylmethoxy-4-methoxybenzaldehyde, under nitrogen protection, then heated in an oil bath to an internal temperature of 100°C, and kept warm at 100°C, the system was gray and turbid, and as time went on, the system became It is dark gray and cloudy. The temperature was maintained at 100° C. for 8 hours, and TLC detected that the reaction was complete.
[0040] Cool down to room temperature and add a solution of 742g sodium hydroxide and 21.7kg deionized water, stir for 15 minutes, filter, and extract 2.7L×3 times with ethyl acetate. While stirring the water phase, add concentrated hydrochloric acid dropwise to adjust pH = 4, extract 4.5 L x 3 times with ethyl acetate, combine the organic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com