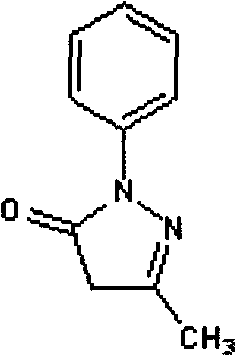
Edaravone medicinal composition for injection and preparation method thereof
A composition and injection technology, applied in the field of medicine, can solve the problems of difficult dissolution of edaravone and poor stability of the pharmaceutical composition, and achieve the effects of simple and easy production method, stable product quality and optimized prescription quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
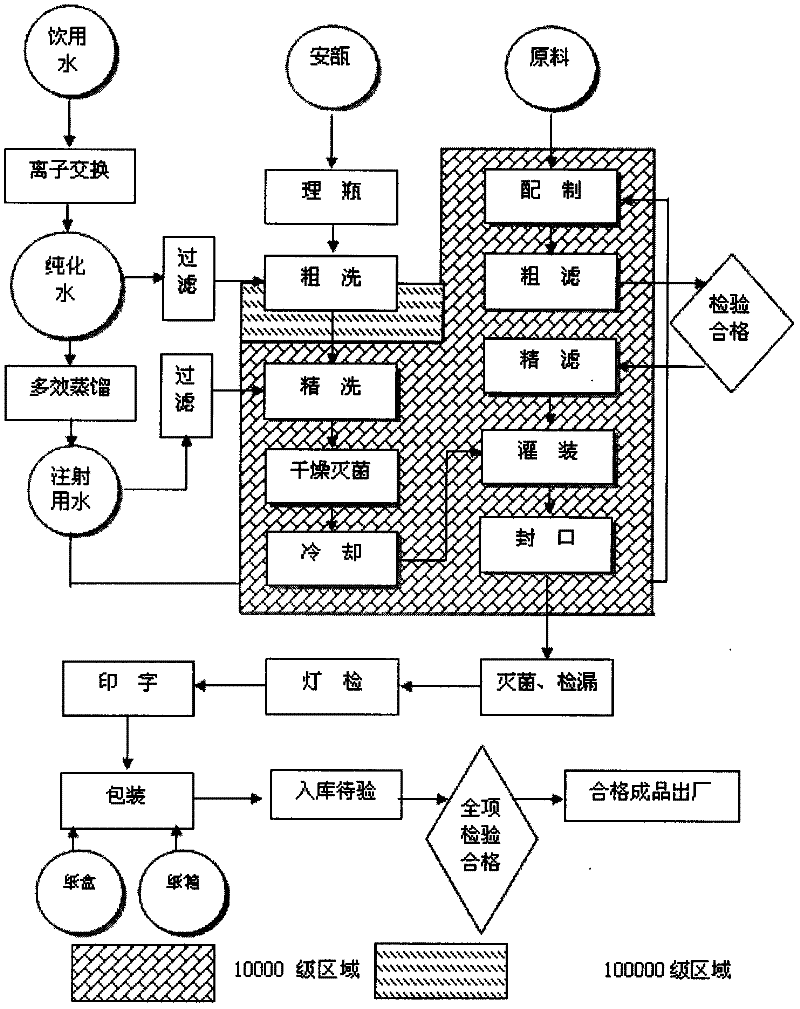
Embodiment 1
[0129] Edaravone injection prepared 1000, wherein:
[0130]
[0131] Preparation Process:
[0132] 1) Preparation: Edaravone is prepared into an amorphous form by spray drying and set aside;
[0133] 2) Take 70% of the prescribed amount of water for injection, at a temperature of 50-70°C, add the prescribed amount of thiobisethyl alcohol, disodium hydrogen phosphate, and sodium chloride, stir until dissolved, and then add the prescribed amount of ida into the solution Lavone, stir until completely dissolved;
[0134] 3) Add 0.05-0.1% medicinal charcoal in the volume of the liquid medicine to item 2), stir well, and place it for 15-30 minutes;
[0135] 4) Filter 3) with a plate and frame filter, add water for injection to the full amount, mix well, measure the initial pH value, adjust the pH value range to 3.0 with 4% sodium hydroxide solution and 10% phosphoric acid solution according to the initial pH value -4.5;
[0136] 5) Central control: conduct intermediate produc...
Embodiment 2
[0143] Edaravone injection prepared 1000, wherein:
[0144]
[0145]
[0146] Preparation process: with embodiment 1.
Embodiment 3
[0148] Edaravone injection prepared 1000, wherein:
[0149]
[0150] Preparation process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com