A new catalytic technique for the preparation of long-chain alkanes from biomass derivatives furfural or hmf
A technology for long-chain alkanes and derivatives, applied in the fields of catalytic chemistry and chemical technology, can solve the problems of high cost, reduced selectivity of target products, and high investment in reaction units
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
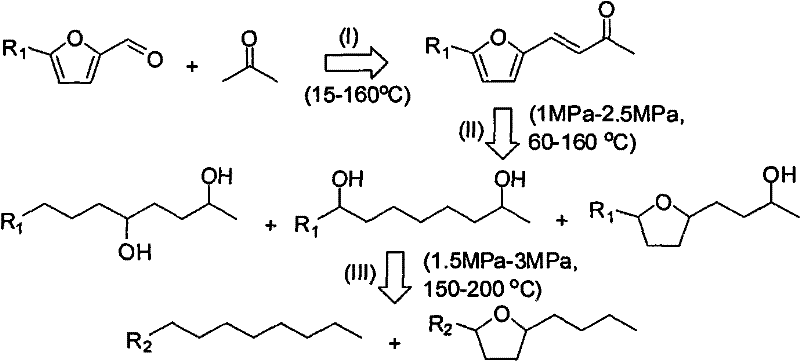
[0016] Mix 9.6g of furfural, 58g of acetone, and 72.4ml of 1.4% NaOH aqueous solution, stir at room temperature for 2 hours, let stand for liquid separation, take the bottom product, wash with a large amount of deionized water, and dry to obtain 11.3g of condensation product. The resulting condensation product was dissolved in 50ml tetrahydrofuran, using Pd / MnO 2 / MgO ring-opening hydrogenation dual-functional catalyst, reacted in a batch reactor, the reaction conditions are: 145 degrees, 2.5MPa, the reaction time is 24 hours, the conversion rate is 99%, 1,7-octanediol selectivity The selectivity for 2,5-octanediol was 35%, and the selectivity for 4-(2-tetrahydrofuryl)-2-butanol was 42%. Finally, the obtained polyol mixture is reacted in a fixed-bed reactor under the action of Pt / HZSM-5 hydrogenation / dehydration dual-function catalyst at 3MPa, 180 degrees, and a space velocity of 2.4h -1 conditions, polyols can be completely converted, octane (C 8 ) selectivity of 45%.
Embodiment 2
[0018] Mix 24g furfural, 100g acetone, and 500ml ethanol evenly, and use 5g MgAl 2 o 4 As a solid base catalyst, it was stirred at 120°C for 20 hours, and the reaction solution was filtered to separate the solid base catalyst. The ethanol solution of the obtained condensation product was directly used as the reaction solution, and Ru / TiO 2 / CeO 2 Ring-opening hydrogenation bifunctional catalyst, reacted in a fixed bed, the reaction conditions are: 160 degrees, 1.5MPa, space velocity 5h -1 , conversion rate is 52%, 1,7-octanediol selectivity is 15%, 2,5-octanediol selectivity is 42%, 4-(2-tetrahydrofuryl)-2-butanol selectivity is 37% %. Finally, the resulting polyol mixture, in Pt / SiO 2 Under the action of hydrogenation / dehydration dual-functional catalyst, the reaction is carried out in a fixed-bed reactor at 2MPa, 200 degrees, and a space velocity of 3.6h -1 Under these conditions, polyols can be completely converted, and the selectivity of octane is 55%.
Embodiment 3
[0020] Mix 24g HMF, 50g acetone, and 500ml ether evenly, and use 10g MgO-ZrO 2 Composite oxide (Mg / Zr=2) was used as a solid base catalyst, stirred at 160°C for 5 hours, and the reaction solution was filtered to separate the solid base catalyst. The diethyl ether solution of the obtained condensation product is directly used as the reaction solution, and the Ni / WO 3 / SiO 2 Ring-opening hydrogenation bifunctional catalyst, reacted in a fixed bed, the reaction conditions are: 60 degrees, 2.5MPa, space velocity 1.2h -1 , conversion rate is 38%, 2,5,9-nonanetriol selectivity is 9%, 1,2,8-nonanetriol selectivity is 18%, 4-(5-hydroxymethyl-2-tetrahydrofuryl )-2-butanol selectivity was 37%. Finally, the obtained polyol mixture, in PtCo / Nb 2 o 5 Under the action of hydrogenation / dehydration dual-functional catalyst, the reaction is carried out in a fixed-bed reactor at 1MPa, 200 degrees, and a space velocity of 10h -1 Under these conditions, polyols can be completely converted, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com