Synthesis method for preparing antihypertensive medicine having benzofuroxan ring
An anti-hypertensive, synthetic method technology, applied in drug combination, organic chemistry, cardiovascular system diseases, etc., can solve the problems of large environmental pollution of chromium ions, cumbersome operation and post-processing, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
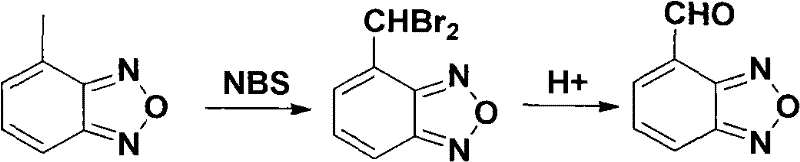
[0028] Synthesis of 4-bromomethylbenzofurazan
[0029] 4-Methylbenzofurazan (13.4g, 100mmol) was dissolved in carbon tetrachloride (150ml), then NBS (23.5g, 132mmol) and Bz 2 o 2 (0.29g, 1.2mmol), the mixture was heated to 80-85°C for reaction, the reaction was completed, the system dropped to 40°C, filtered, filtered and washed with chloroform, and the solvent was recovered under reduced pressure to obtain a crude product (25.6g), petroleum ether-acetic acid Ethyl ester was recrystallized to obtain light yellow needle crystals, 4-bromomethylbenzophenazine (15.3g). Yield 77.6%, mp 94-95°C.
Embodiment 2
[0031] 4-Formylbenzofurazan
[0032] 4-Bromomethylbenzofurazan (19.7 g, 0.1 mol), DMSO 15 ml, and sodium bicarbonate (10 g, 0.12 mol) were added to the reaction flask. Heated to 100-150°C under nitrogen protection. After the reaction was completed, it was cooled to room temperature, extracted with water and ethyl acetate. The organic layers were combined and washed with saturated brine. Dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain 10.6 g of light yellow solid, yield 71.6%, mp 108-109°C.
Embodiment 3
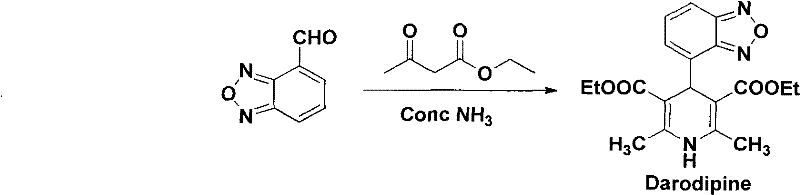
[0034] Synthesis of Darrodipine
[0035] Add 4-formylbenzofurazan (14.8g, 0.1mol), ethyl acetoacetate (28g, 0.2mol), ammonium carbonate (15.8g, 0.2mol) in the 500mL three-neck round bottom flask, under stirring at 70 ℃ reaction, TLC tracking. After the reaction was finished, 200ml of water was added, and the reaction mixture was extracted 3 times with 200mL of ethyl acetate, the organic layers were combined, washed with water, dried, and the solvent was recovered under reduced pressure, cooled and crystallized, and recrystallized to obtain 29.5g of yellow solid darodipine, the yield 79.5%, mp 153-154°C. 1 H-NMR (CDCl 3 400MHz) δ: 7.61(m, 1H, Ar-H), 7.29(m, 2H, Ar-H), 5.98(s, 1H, N-H), 5.49(s, 1H, Ar-CH), 4.04(q, J=7.2Hz, 4H, COOCH 2 ), 2.32(s, 6H, 2×CH 3 ), 1.13(t, J=7.2Hz, 6H, COOCH 2 CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com