Benzoselenadiazole derivatives with anti-tumor and anti-oxidation activities and their preparation and application
A technology of antioxidant activity and benzoselenodiazole, which is applied in the field of medicine to achieve the effects of high yield, simple preparation method, good anti-tumor and antioxidant activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
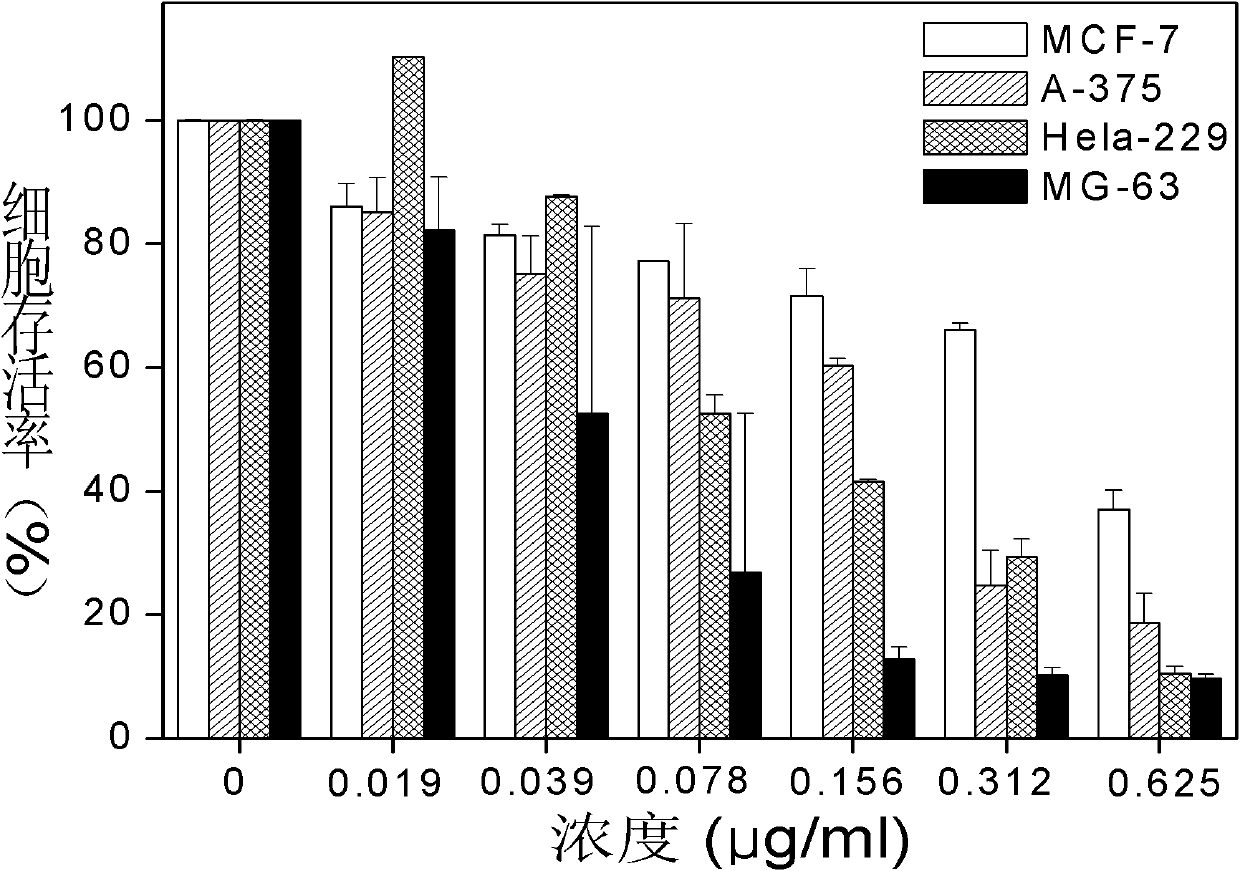
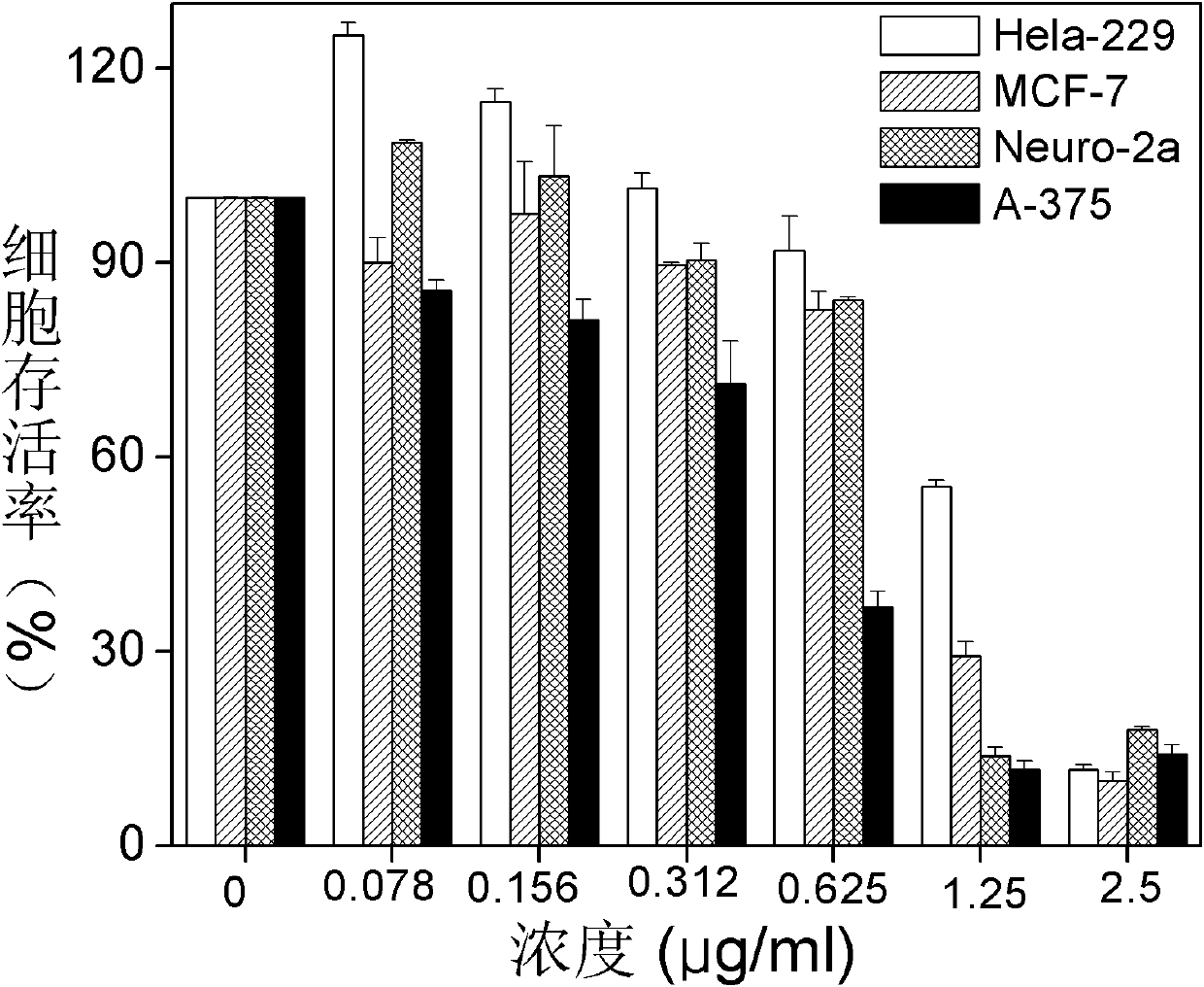
Examples
Embodiment 1
[0039] Example 1: Preparation of 5-nitrobenzo[c][1,2,5]selenodiazole (abbreviated as NS)
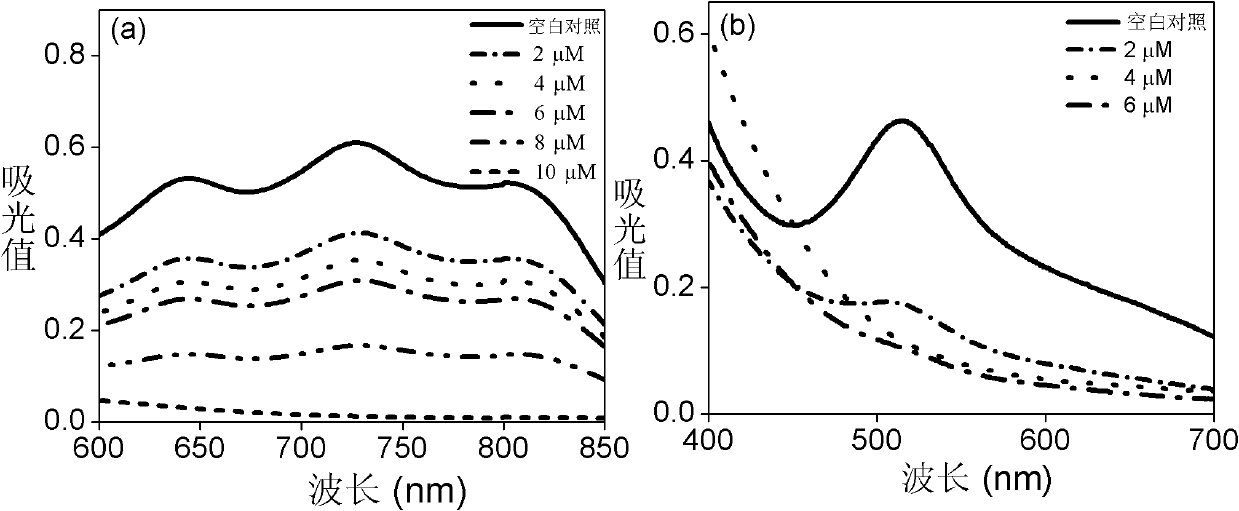
[0040] Add 1 mmol 4-nitro-o-phenylenediamine to a three-necked flask, then add 95% (v / v) ethanol to dissolve it completely, heat and stir, and add an equimolar amount of SeO dissolved in a little water drop by drop. 2 , heated to 85° C. and refluxed, reacted for 3 hours and precipitated a large amount of khaki precipitate, filtered it with suction, washed three times with 95% ethanol, and dried to obtain a khaki solid with a yield of 80% (mass percentage). ESI-MS (CH 3 OH): m / z 227.1 ((M-H), 100%). 1 H NMR (DMSO): δ8.8(s 1H), δ8.2(m 1H), 8.1(d 1H). IR characteristic peak (KBr), υ / cm -1 : 449, 527, 560 (υ N-Se-N ).UV-Vis(DMSO):λ max 351nm. Fluorescence emission peak (DMSO and water): λ max 689nm.
[0041]
Embodiment 2
[0042] Example 2: Preparation of 5,6-dinitrobenzo[c][1,2,5]selenodiazole (abbreviated as DNS)
[0043] Take 1mmol of 4,5-dinitro-o-phenylenediamine in an agate mortar, then add 1mmol of SeO that is ground into fine powder 2 Solid, then grind at room temperature for half an hour, then transfer the ground mixture to 95% (v / v) ethanol solution, heat to dissolve the precipitate completely, heat filter to remove insoluble matter, and the filtrate precipitates yellow-gray needles after cooling Crystal, yield 80% (mass percentage). ESI-MS (CH 3 OH): m / z 274.2 ((M-H)-, 100%). 1 H NMR (DMSO): δ9.2 (m 1H), δ9.0 (m 1H). IR characteristic peak (KBr), υ / cm -1 : 437,583(υ N-Se-N ).UV-Vis(DMSO):λ max 364nm.
[0044]
Embodiment 3
[0045] Example 3: Preparation of 5-(3,4-diaminobenzene)-benzo[c][1,2,5]selenadiazole (abbreviated as BSBD)
[0046] Weigh 2mmol 3,3′,4,4′-biphenyltetramine in a single-necked flask, add 1.5mol / L hydrochloric acid solution to dissolve it completely, add 1mmol SeO2 dropwise at room temperature 2 Aqueous solution, after reacting for 2 hours, filter to remove impurities, adjust the filtrate to pH 8 with saturated NaOH solution under the condition of stirring in an ice bath, precipitate a gray precipitate of soil, filter with suction, wash the obtained solid several times with a small amount of water, and then dry it in vacuum to obtain The desired product, the yield is 60% (mass percentage). ESI-MS (CH 3 OH): m / z 291.1 ((M+H) + , 100%). 1 H NMR (DMSO): δ7.8(m1H), δ7.0(m 1H), δ6.6(m 1H), δ4.8(s 1H), δ4.6(s 1H), δ3.3(d 1H).IR characteristic peak (KBr), υ / cm -1 : 455,671(υ N-Se-N ).UV-Vis(DMSO):λ max 467nm. Fluorescence emission peak (DMSO and water): λ max 669nm.
[0047]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com