A Strain of Enterococcus Faecalis for Feed and Its Application
A technology of Enterococcus faecalis and Escherichia coli, which is applied in the fields of application, bacteria, animal feed, etc., to achieve the effects of enhancing animal disease resistance, reducing feed-to-meat ratio, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
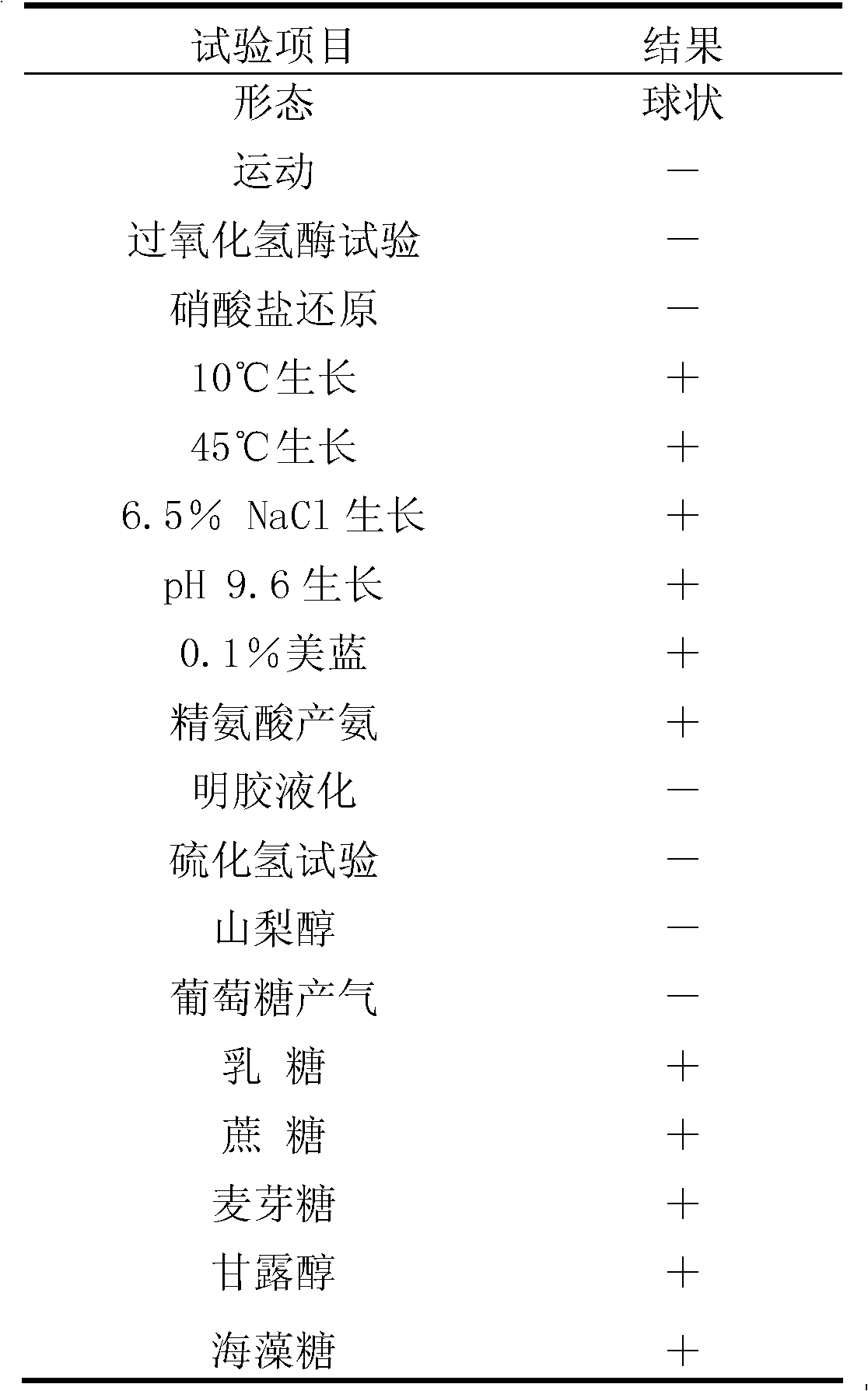
[0032] Example 1. Isolation, Identification and Preservation of Enterococcus faecalis SBD CGMCC No.4848
[0033] 1. Isolation of Enterococcus faecalis SBD CGMCC No.4848
[0034] MRS liquid medium: glucose 20g / L, peptone 10g / L, yeast extract 5g / L, diammonium citrate 2g / L, sodium acetate 5g / L, beef extract 10g / L, Tween 801g / L, K 2 HPO 4 2g / L, MgSO 4 ·7H 2 O0.058g / L, MnSO 4 4H 2 O 0.25g / L, pH 6.8.
[0035] Enrichment medium: liquid MRS medium containing 40 μg / mL cycloheximide.
[0036] CaCO 3 MRS medium: MRS solid medium containing 0.5% (mass percentage) light calcium carbonate.
[0037] Acid-resistant MRS medium: Adjust the pH of the MRS liquid medium to 3.0.
[0038] Bile salt-tolerant screening medium: add 0.3% (mass percentage) pig bile salt to the MRS medium.
[0039] The fresh feces of sows were collected from the Breeding Pig Testing Center of Huazhong Agricultural University, enriched with enriched culture medium (the culture condition was 37°C, static culture),...
Embodiment 2
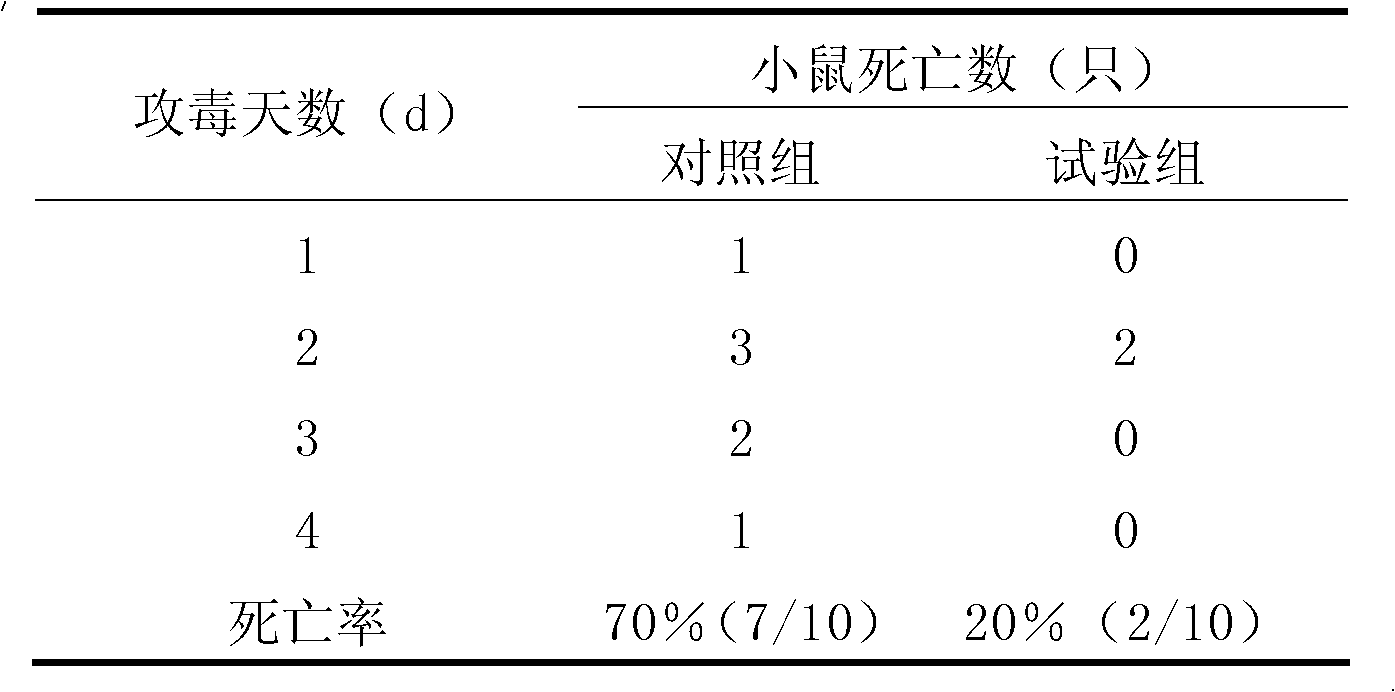
[0048] Embodiment 2, analysis of the gastric juice resistance property of Enterococcus faecalis (Enterococcus faecalis) SBD CGMCC No.4848
[0049] Preparation of synthetic gastric juice: tryptone 8.3g, glucose 3.5g, sodium chloride 2.05g, calcium chloride 0.11g, potassium chloride 0.37g, potassium dihydrogen phosphate 0.6g, pig bile salt 0.05g, lysozyme 0.1g, stomach Add 13.3 mg of protease and 1000 mL of distilled water, adjust the pH values to 1.5 and 2.5 with 1 mol / L hydrochloric acid, and sterilize at 115°C for 20 minutes. Among them, lysozyme and pepsin were sterilized by filtration.
[0050] To activate the strain, transfer Enterococcus faecalis (Enterococcus faecalis) SBD CGMCCNo.4848 into 100mL liquid MS medium according to the inoculum size of 1%, cultivate overnight at 37°C and 200r / min, take 1mL of bacterial liquid and add 9mL of aseptic synthetic In the gastric juice, the number of viable bacteria was detected by plate counting method at 0 min, 30 min, 60 min, a...
Embodiment 3
[0052] Example 3, analysis of bile salt resistance properties of Enterococcus faecalis (Enterococcus faecalis) SBD CGMCC No.4848
[0053] Prepare MRS liquid medium, which is added with 0.1%, 0.3%, 0.5%, 1.0%, 2.0%, 3.0%, 5.0% (mass percentage concentration) bile salt (purchased from Sinopharm Pharmaceutical Co., Ltd.). Take 1mL 37 ℃ overnight cultivation of Enterococcus faecalis (Enterococcus faecalis) SBD CGMCC No.4848 bacterial cell fermentation broth (10 9 CFU / mL), added to 9mL MRS liquid medium supplemented with the above concentrations of bile salts, cultured at 37°C for 12h, diluted 10 4 After doubling, take 0.1mL solid agar plate coated with MRS, and observe the growth of the bacteria to detect its bile salt tolerance. If the number of growth exceeds 30 (the number of bacteria is 3×10 6 CFU / mL) indicates that it grows well and can tolerate the concentration of bile salts; if the growth number is 5-30, it indicates that the growth is poor, and the ability to tolerate t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com