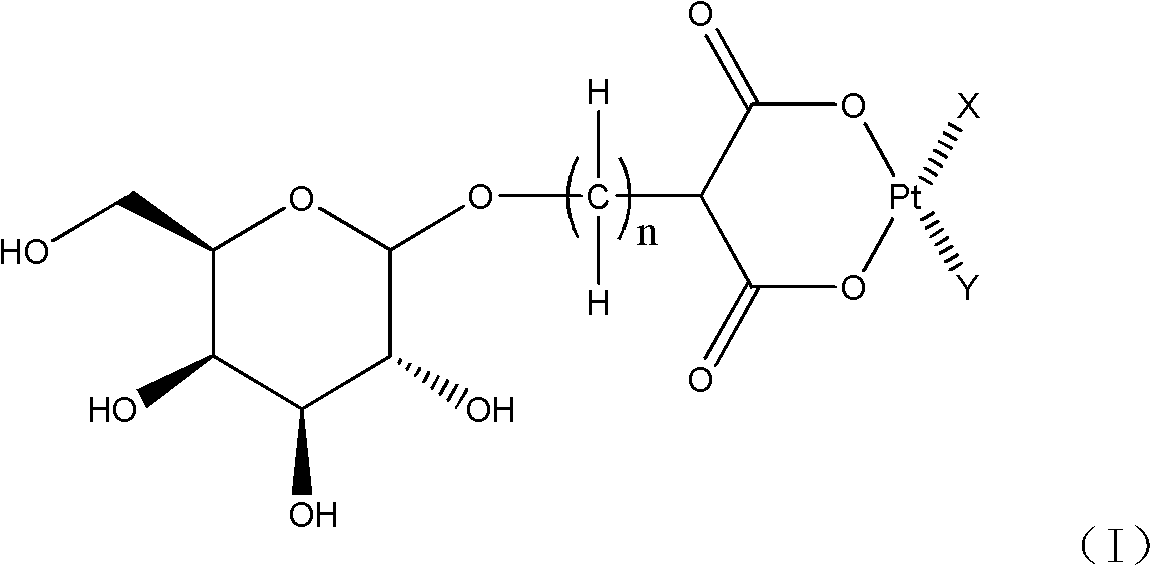
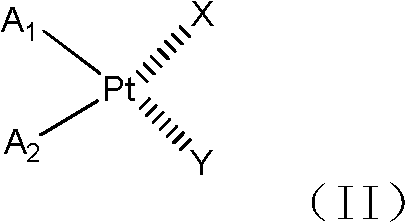
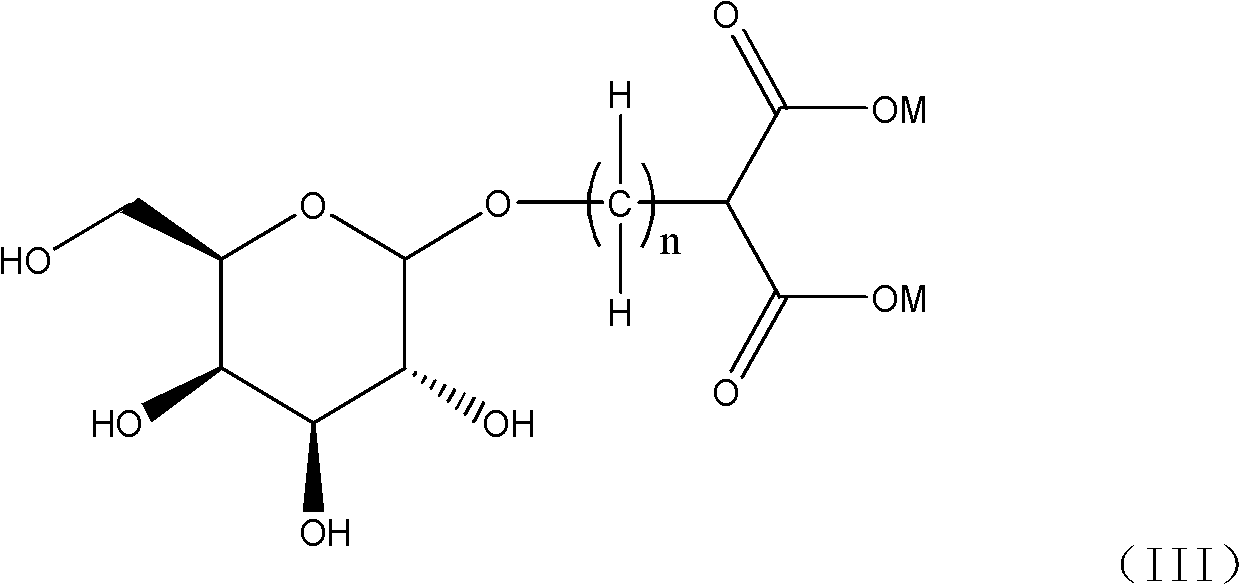
Galactose-containing platinum complex for tumour targeted therapy and preparation method thereof
A technology of tumor targeting and platinum complexes, which is applied in the preparation of sugar derivatives, medical preparations containing active ingredients, sugar derivatives, etc., which can solve the problems of increasing toxic side effects, difficult renal elimination, and lack of tumor targeting function of drugs and other problems, to achieve the effect of high water solubility and clinical application of tumor targets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The specific preparation can be accomplished using the following methods and reaction formulas.
[0050] Method A:
[0051]
[0052] Method B:
[0053]
[0054] In method A, when M is a hydrogen atom in formula (III), the reaction can be carried out by using an appropriate inorganic base, such as sodium hydroxide, potassium hydroxide, sodium carbonate, sodium bicarbonate, potassium carbonate, lithium hydroxide and hydrogen cesium oxide etc. to adjust the pH of the reaction aqueous solution and maintain between 7-9 to complete the preparation of the compound shown in formula (I); when M is a metal atom, for example: sodium atom, potassium atom, lithium atom, barium atom or cesium Atoms, the reaction can be carried out smoothly directly in the aqueous solution, and if necessary, use a small amount of the aqueous solution of the above-mentioned inorganic base to maintain the pH of the reaction solution between 7-9 to complete the synthesis of the complex shown in fo...
Embodiment 1
[0076] (1) Preparation of 1-O-D-galactoside-2-bromo-ethane:
[0077]
[0078] 1) Add galactose (2.7g, 15mmol) to 2-bromoethanol (10ml) at room temperature, cool to 0°C, replace the air in the flask with nitrogen, and slowly add boron trifluoride dropwise under nitrogen protection Ether solution (98%, 1 ml).
[0079] 2) Stir the reaction solution at 0°C for 15 minutes, then slowly warm up to room temperature and stir for 30 minutes, then heat the reaction solution to 80°C, and react at 80°C for 5 hours.
[0080] After the reaction was completed, the solvent was removed by rotary evaporation, and the reaction product was simply purified by silica gel column chromatography (dichloromethane:methanol, 6:1) to obtain 2.4 g of a crude product.
[0081] Mass spectrum: MS, m / z: 287.03 [M+H] +
[0082] (2) Preparation of 1-O-(2,3,4,6-tetraacetyl-D-galactoside)-2-bromo-ethane:
[0083]
[0084] At room temperature, 2.4 g of the product 1-O-D-galactoside-2-bromo-ethane obtained ...
Embodiment 2
[0133] (1) Preparation of 1-O-D-galactoside-3-bromo-propane:
[0134]
[0135] Add galactose (1.8g) to 3-bromopropanol (10mL) at room temperature, cool to 0°C, replace the air in the flask with nitrogen, and slowly drop in the ether solution of boron trifluoride under the protection of nitrogen (98%, 1 mL). The reaction solution was stirred at 0°C for 15 minutes, raised to room temperature and stirred for 30 minutes, then heated to 80°C, and reacted at 80°C for 5 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and 2.05 g of crude product was obtained after simple purification by silica gel column chromatography (dichloromethane:methanol, volume ratio 6:1).
[0136] Mass spectrum: MS, m / z: 301.03 [M+H] +
[0137] (2) Preparation of 1-O-(2,3,4,6-tetraacetyl-D-galactoside)-3-bromo-propane:
[0138]
[0139] At room temperature, 2.05 g of the crude product of 1-O-D-galactoside-3-bromo-propane obtained in the previous step was disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com