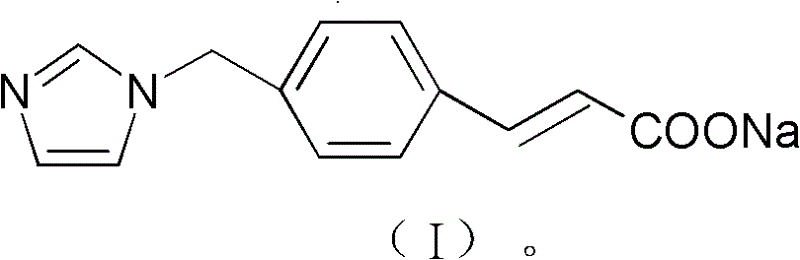
New ozagrel sodium compound and medicinal composition thereof
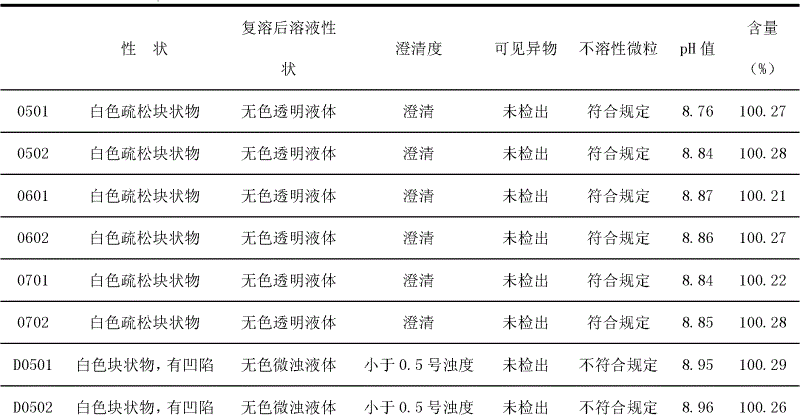
The technology of sodium ozagrel and compound is applied in the field of new sodium ozagrel and pharmaceutical compositions thereof, and can solve the problems of colored spots, color blocks, poor resolubility of lyophilized powder for injection, suspended particles and the like in solution, Achieving the effect of good shape, good resolubility and good appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 ozagrel sodium crystalline compound
[0039] (1) Dissolve the ozagrel sodium solid in an ethanol solution with a concentration of 60% by volume, and filter; add 20ml of 60% ethanol to each gram of ozagrel sodium compound;
[0040] (2) Add a mixed solvent of anhydrous acetone and anhydrous methanol at 35°C, and stir while adding; the volume ratio of the volume sum of the added anhydrous acetone and anhydrous methanol to the ethanol solution is 1.5:1, without The volume ratio of water acetone and anhydrous methanol is 1:4; the speed of anhydrous acetone and anhydrous methanol is 1ml / min, and the stirring speed is 30 rpm;
[0041] (3) Cool down to 10°C after adding the mixed solvent, and let stand for 1 hour;
[0042] (4) The crystals were collected, washed with absolute ethanol, and vacuum-dried for 3 hours to obtain ozagrel sodium crystals.
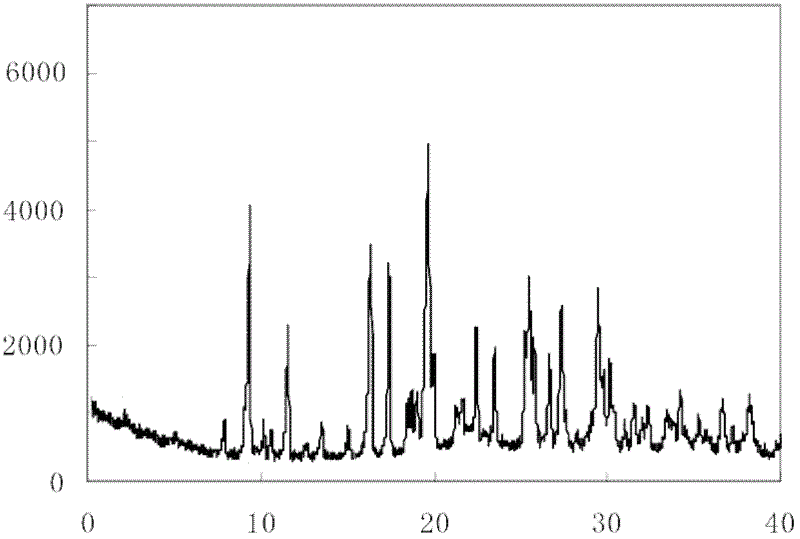
[0043] The prepared X-ray powder diffraction measured by Cu-Kα ray is 9.4°, 11.5°, 16.4°, 17.5°, 1...
Embodiment 2
[0044] The preparation of embodiment 2 ozagrel sodium crystals
[0045] (1) Dissolve the solid sodium ozagrel in an ethanol solution with a concentration of 80% by volume, and filter; add 100 ml of ethanol to each gram of sodium ozagrel compound;
[0046] (2) Add a mixed solvent of anhydrous acetone and anhydrous methanol at 40°C, and stir while adding; the volume ratio of the volume sum of the added anhydrous acetone and anhydrous methanol to the ethanol solution is 0.5:1, without The volume ratio of water acetone and anhydrous methanol is 1:2.75; the speed of adding dropwise of anhydrous acetone and anhydrous methanol is 2ml / min; the stirring speed is 60 rev / min;
[0047] (3) Cool down to 15°C after adding the mixed solvent, and let stand for 1 hour;
[0048] (4) The crystals were collected, washed with absolute ethanol, and vacuum-dried for 3 hours to obtain ozagrel sodium crystals.
[0049] The X-ray powder diffraction of the prepared sodium ozagrel crystals measured by ...
Embodiment 3
[0050] The preparation of embodiment 3 ozagrel sodium crystals
[0051] (1) Dissolve the solid sodium ozagrel in an ethanol solution with a concentration of 75% by volume, and filter; add 80 ml of ethanol to each gram of sodium ozagrel compound;
[0052] (2) Add a mixed solvent of anhydrous acetone and anhydrous methanol at 35°C, and stir while adding; the volume ratio of the volume sum of the added anhydrous acetone and anhydrous methanol to the ethanol solution is 1.5:1, without The volume ratio of water acetone and anhydrous methanol is 1:3.5; the speed of adding dropwise of anhydrous acetone and anhydrous methanol is 1.5ml / min; the stirring speed is 90 rpm;
[0053] (3) Cool down to 10°C after adding the mixed solvent, and let stand for 1 hour;
[0054] (4) The crystals were collected, washed with absolute ethanol, and vacuum-dried for 3 hours to obtain ozagrel sodium crystals.
[0055] The X-ray powder diffraction pattern obtained by measuring the prepared sodium ozagre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com