A kind of composite functional fluoride removal resin and its preparation method
A composite function, fluorine-removing resin technology, applied in chemical instruments and methods, other chemical processes, adsorption water/sewage treatment, etc., to achieve high adsorption capacity and wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (a) Nitrification reaction
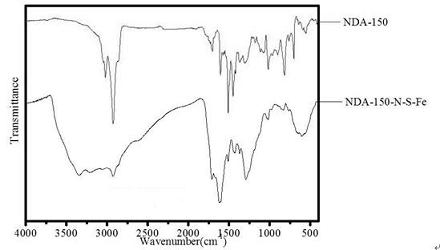
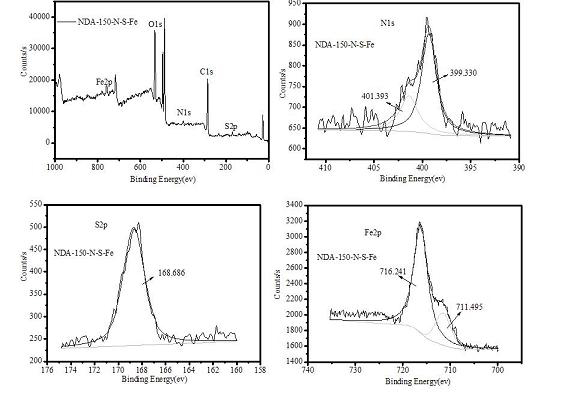
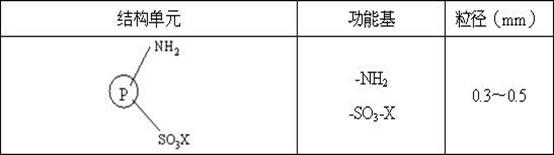
[0027] Put styrene-divinylbenzene resin NDA-150 into a Soxhlet extractor, extract it with ethanol, air-dry it, sieve it, and take the resin with a suitable particle size for use.
[0028] In a 500 mL beaker, add 40 ml of 65% concentrated nitric acid and 80 ml of 85% concentrated sulfuric acid, stir well and cool for later use.
[0029] In a 500 mL three-neck flask, add 10 g of NDA-150 resin, pour into the above mixed solution, stir and react at 50 °C for 0.5 h, after the reaction, pump out the reaction solution, pour it into the ice-water mixture, filter and rinse with distilled water until Neutral, air drying to get resin 150-NO 2 .
[0030] (b) Sulfonation reaction
[0031] In a 500 mL three-neck flask, add the 150-NO prepared in step (a) 2 Resin 10 g, swollen with 20 ml of dichloroethane for 6 h, added 80 ml of 80% concentrated sulfuric acid, then refluxed and heated to 90 ° C, stirred, reacted for 8 h, after the end, distilled dichlo...
Embodiment 2
[0040] In step (a), the concentration of concentrated nitric acid is 60% by weight, and the dosage is 10 times of the amount of resin; the concentration of concentrated sulfuric acid is 98% by weight, and the dosage is 8 times of the amount of resin; the reaction temperature is 40° C., and the reaction time is 2 hours; In step (b), use 40ml of dichloroethane to swell for 9 hours, the concentration of concentrated sulfuric acid is 80% by weight, the amount used is 20 times the amount of resin, the reaction temperature is 70 ° C, and the reaction time is 12 hours; in step (c), SnCl 2 2H 2 The purity of O is 90%, and the dosage is 10 times of the amount of resin; the concentration of concentrated hydrochloric acid is 30% by weight, and the dosage is 10 times of the amount of resin; the purity of ethanol is 90%, and the dosage is 15 times of the amount of resin, and the reaction temperature is 80°C , the reaction time is 12h; step (d) Fe 2 (SO 4 ) 3 The weight percentage concen...
Embodiment 3
[0042] In step (a), the concentration of concentrated nitric acid is 80% by weight, and the dosage is 4 times of the amount of resin, the concentration of concentrated sulfuric acid is 75%, and the dosage is 20 times of the amount of resin, the reaction temperature is 80°C, and the reaction time is 3h; In step (b), use 50ml of dichloroethane to swell for 12h, the concentration of concentrated sulfuric acid is 98% by weight, the amount used is 8 times the amount of resin, the reaction temperature is 120°C, and the reaction time is 16h; in step (c), SnCl 2 2H 2 The purity of O is 100%, and the dosage is 4 times of the amount of resin; the concentration of concentrated hydrochloric acid is 40% by weight, and the dosage is 4 times of the amount of resin; ethanol is absolute ethanol, and the dosage is 5 times of the amount of resin, and the reaction temperature is 100°C , the reaction time is 16h; step (d) Fe 2 (SO 4 ) 3 The weight percentage concentration is 1.0 mol / L, the dosa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com