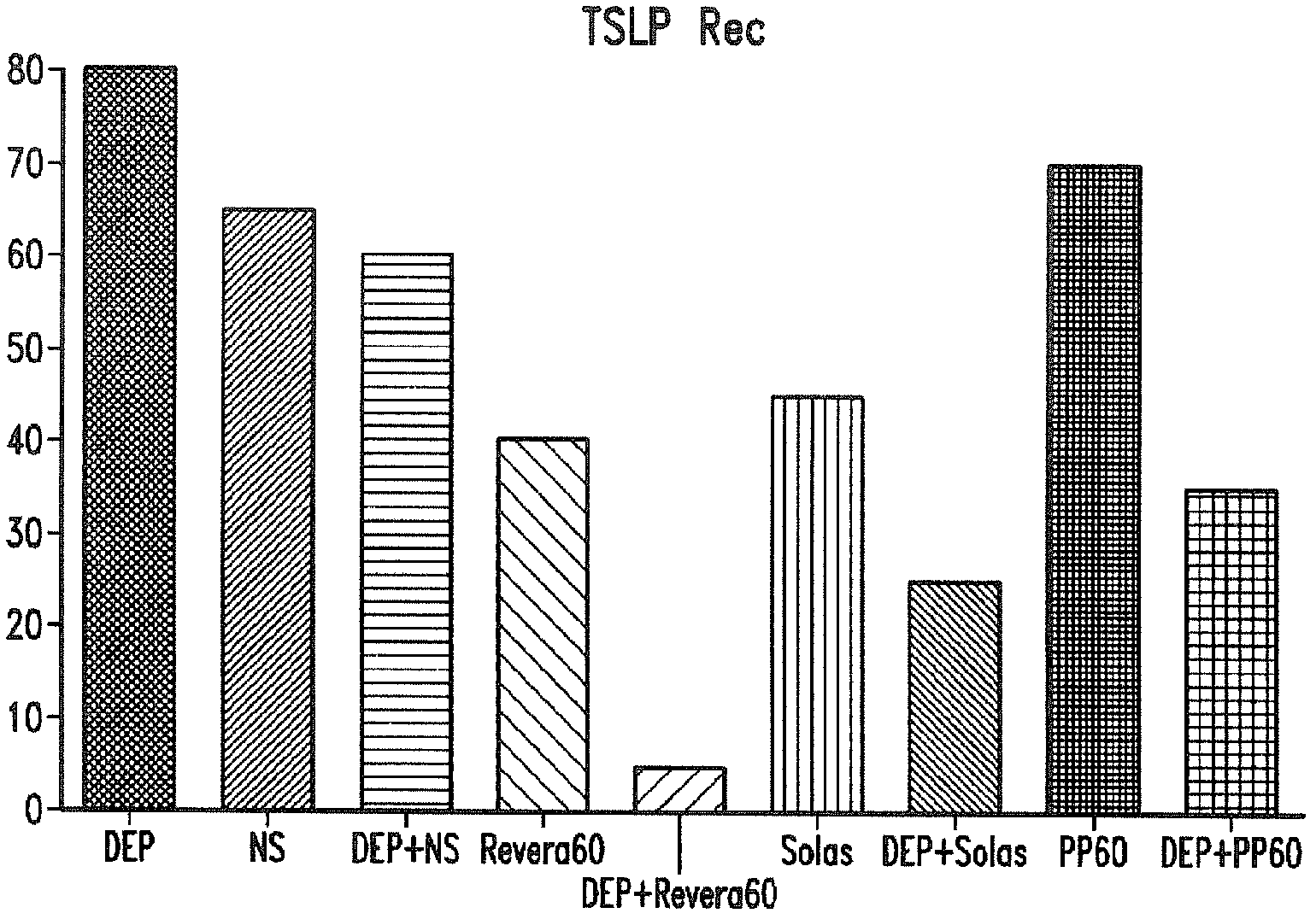
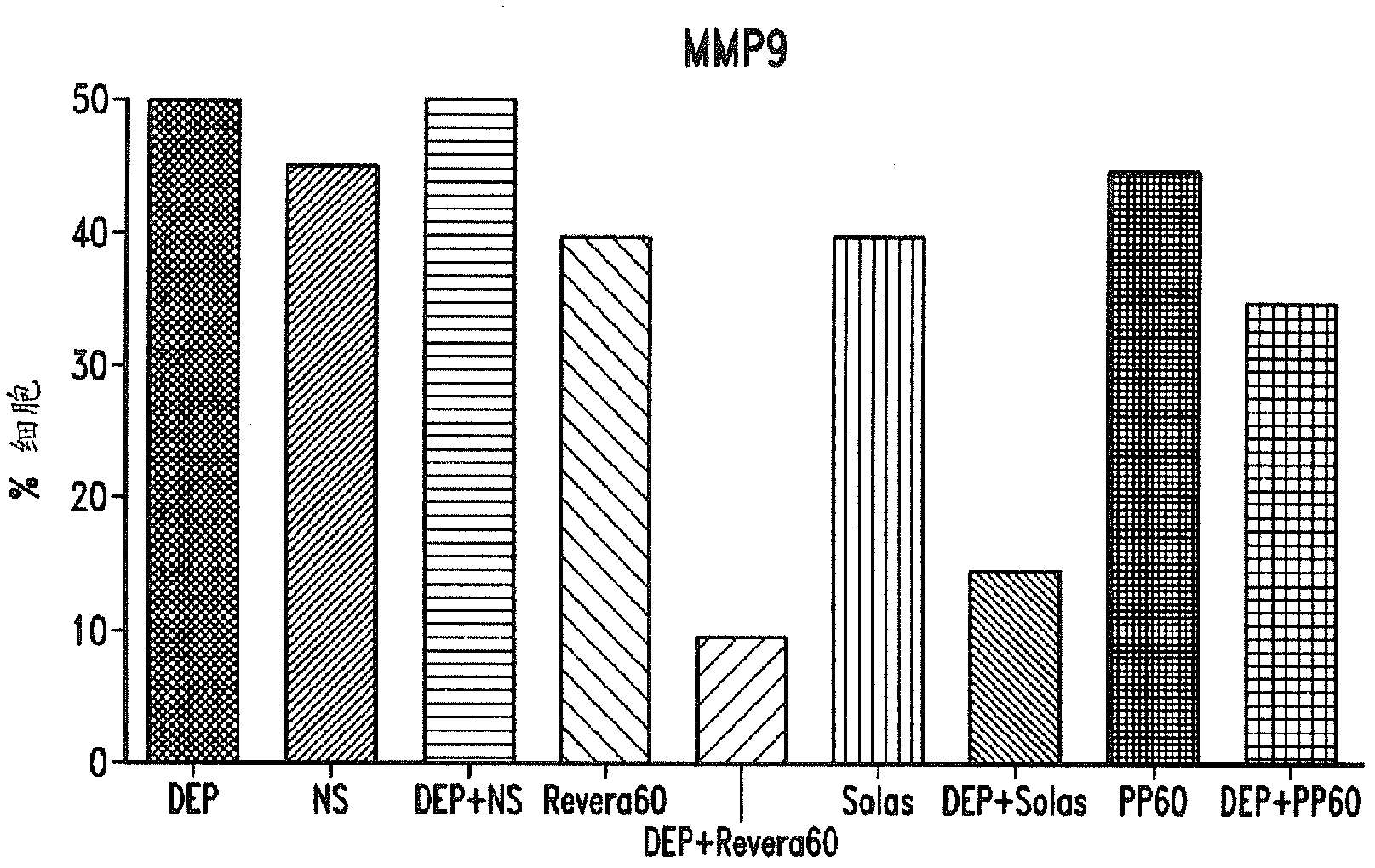
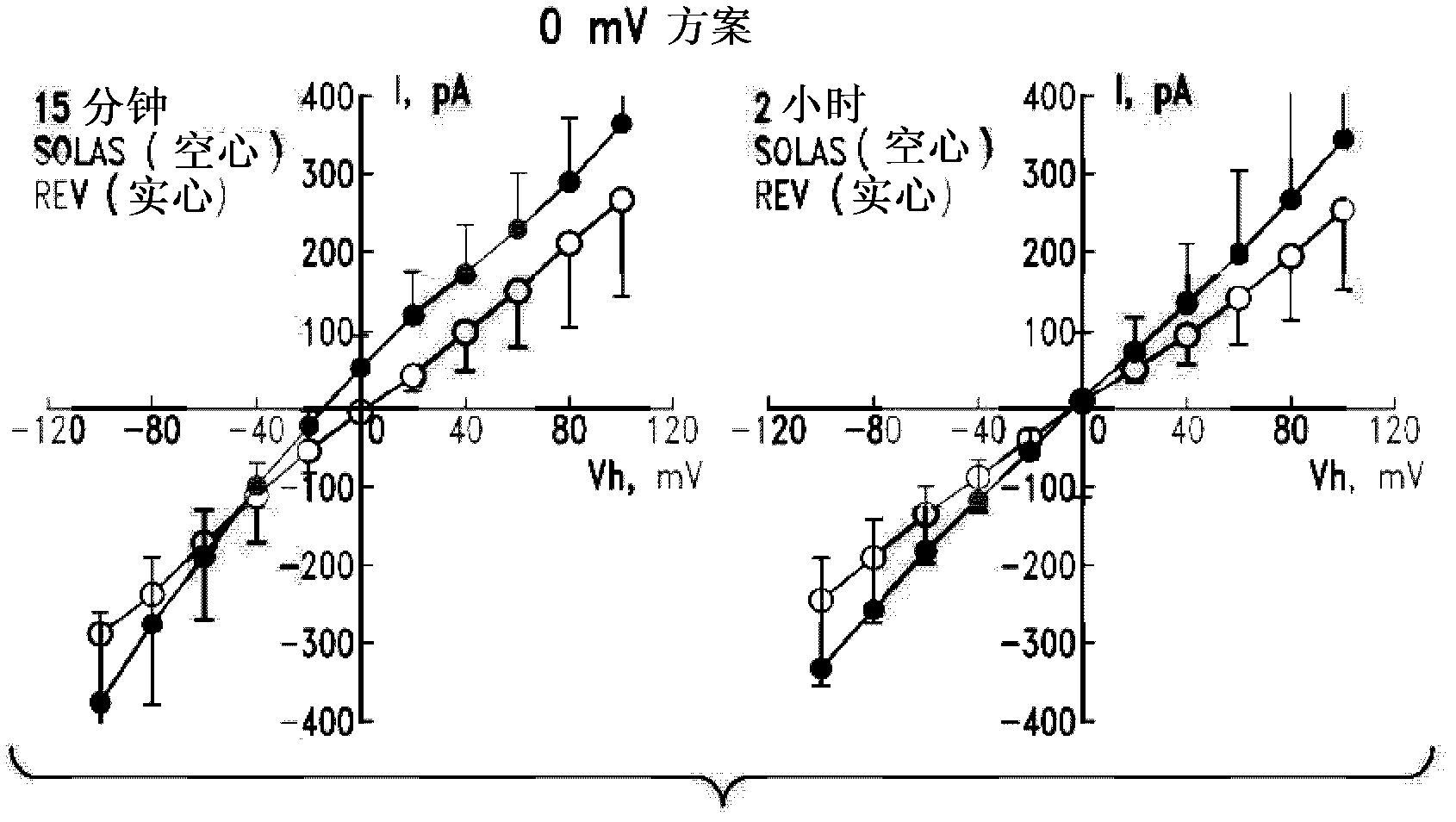
Compositions and methods for treating thymic stromal lymphopoietin (tslp)-mediated conditions
A technology of mediation and disease, applied in the direction of biochemical equipment and methods, microorganisms, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] (Demonstrating the synergistic effect of the electrokinetic fluid of the present invention and albuterol)
[0171] review. In an art-recognized animal model of human bronchoconstriction (human asthma model), the electrokinetic-altered fluids of the present invention and albuterol provide a synergistic prolongation in vivo (e.g., inhibition of bronchoconstriction), thereby reducing the patient's response to Albuterol use. The results disclosed in this example are also disclosed in the applicant's WO 2009 / 055729.
[0172] First test. In the first experiment, the effect of bronchodilators on airway function in sixteen guinea pigs was assessed in combination with methacholine-induced bronchoconstriction. After determining optimal dosing, each animal was dosed at 50 μg / mL to deliver a target dose of 12.5 μg of albuterol sulfate per animal in 250 μL. The study was a randomized block design for weight and baseline PenH values. Two groups (A and B) received an intratrachea...
Embodiment 2
[0183] (Determining the Effect of the Electrokinetic Altered Fluids of the Invention on Cytokine Expression)
[0184] review. When compared to control fluids, the electrokinetic altered fluids of the present invention reduced pro-inflammatory cytokines (IL-1β, TNF-α, IL-6 and GM-CSF), chemokines (IL-8, MIP- 1α, RANTES and Eotaxin), inflammatory enzymes (iNOS, COX-2 and MMP-9), allergen response (MHC class II, CD23, B7-1 and B7-2) and production of Th2 cytokines (IL-4, IL-13, and IL-5); and increased anti-inflammatory cytokines (eg, IL1R-α, TIMP) compared to control fluids. The results disclosed in this example are also disclosed in the applicant's WO 2009 / 055729.
[0185] In specific aspects, human mixed lymphocytes were stimulated with T3 antigen or PHA in Revalesio oxygen-enriched fluid or control fluid, and IL-1β, IL-2, IL-4, IL-5, IL-6 were assessed , IL-7, IL-8, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-17, eotaxin, IFN-γ, GM-CSF , MIP-1β, MCP-1, G-CSF, FGFb, VEGF, TNF...
Embodiment 3
[0204] (Determining the Effect of the Inventive Electrokinetic Altered Fluids on Modulating T Cell Proliferation)
[0205] review. The electrokinetic-altered fluids of the present invention improved regulatory T cell function as shown by relatively reduced proliferation. The results disclosed in this example are also disclosed in the applicant's WO 2009 / 055729.
[0206] The ability of specific embodiments disclosed herein to modulate T cells was investigated by irradiating antigen presenting cells and introducing antigen and T cells. Typically, these stimulated T cells proliferate. However, normal T cell proliferation was suppressed when regulatory T cells were introduced.
[0207] method. Briefly, FITC-conjugated anti-CD25 (ACT-1 ) antibody used in sorting was purchased from DakoCytomation (Chicago, IL). Other antibodies used were as follows: CD3 (HIT3a in soluble condition), GITR (PE conjugated), CD4 (Cy-5 and FITC conjugated), CD25 (APC conjugated), CD28 (CD28.2 clone ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com