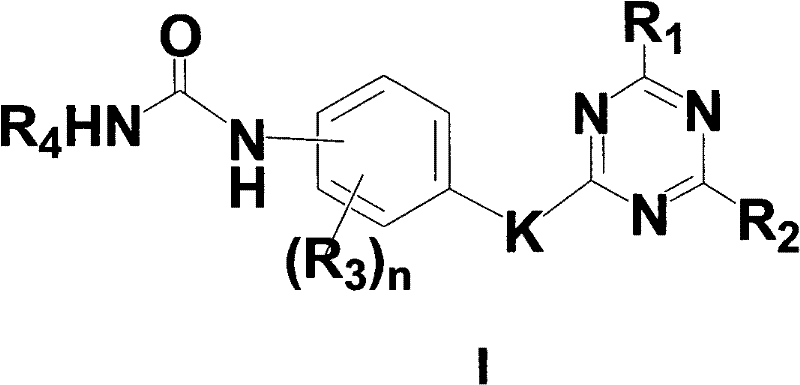
Substituted triazine phenyl urea derivatives and application thereof
A kind of technology of substituent and compound, applied in the field of substituted triazine phenylurea derivatives and their uses, can solve problems such as no effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1: Compound 1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(3-(4-(4-hydroxypiperidin-1-yl)-6-methyl-1 Of, 3,5-triazinyl-2-amino)-4-methylphenyl)urea (HSCA-001)
[0135] Synthetic Route 1
[0136]
[0137] step one:
[0138]
[0139] Dissolve 1a (3.69g, 20mmol) in 20mL of anhydrous tetrahydrofuran, and slowly add methylmagnesium bromide in ether (8mL, 24mmol, 3M) with a syringe at -20°C. After the addition, the temperature was raised to room temperature, and the solvent was concentrated under reduced pressure. Add 20 mL saturated ammonium chloride solution to the residue, extract the solution with ethyl acetate (20 mL×2), wash the organic phase with 30 mL brine, combine the organic layers, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure. Obtained 3.2 g of solid, product 1b, with a yield of 97%. MS[M+1] + 165.2.
[0140] Step two:
[0141]
[0142] 1c (1.11g, 5mmol), 1d (837mg, 5.5mmol) and diisopropylethylamine (1.65mL) were a...
Embodiment 2
[0152] Example 2: Compound 1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-methyl-3-(4-methyl-6-morpholinyl-1,3, Synthesis of 5-triazinyl-2-amino)phenyl)urea (HSCA-002)
[0153] Synthetic Route 2
[0154]
[0155] 1g (150mg, 0.319mmol) and morpholine (1mL) were added to 10mL ethanol solution and stirred at room temperature for 3 hours. The solvent was concentrated under reduced pressure, water was added, extracted with ethyl acetate, the organic phase was collected and concentrated. Purified with a preparative silica gel plate to obtain 60 mg of 1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-methyl-3-(4-methyl-6-morpholinyl-1) , 3,5-triazinyl-2-amino)phenyl)urea (HSCA-002), the yield is 36%. 1 H NMR(400MHz, DMSO-d 6 )δppm: 9.07 (s, 1H), 8.76 (s, 1H), 8.72 (s, 1H), 8.14 (s, 1H), 7.80 (s, 1H), 7.59 (m, 2H), 7.10 (m, 1H) ), 7.03(m, 1H), 3.68(m, 4H), 3.58(m, 4H), 2.20(s, 3H), 2.15(s, 3H); MS[M+1] + 522.0.
[0156] The following compounds can be obtained by the methods of Examples 1 and 2:
...
Embodiment 3
[0212] Example 3: Compound 1-(4-chloro-3-(trifluoromethyl)phenyl)-3-(4-methyl-3-(4-methyl-6-morpholinyl-1,3, Synthesis of 5-triazinyl-2-oxo)phenyl)urea (HSCA-003)
[0213] Synthetic Route 3
[0214]
[0215] step one:
[0216]
[0217] 3a (1.3 g, 7.9 mmol) and diisopropylethylamine (1.6 g, 15.8 mmol) were dissolved in 15 mL DMF, and morpholine (620 mg, 0.71 mmol) was added thereto at -5°C. After stirring for 2 hours at the same temperature, water was added to the reaction solution, extracted with ethyl acetate, and the organic phase was collected, dried with anhydrous sodium sulfate and filtered. The solvent was concentrated under reduced pressure to obtain 1.4 g of crude product 3b, which was directly used in the next reaction.
[0218] Step two:
[0219]
[0220] Compound 3c (544 mg, 3.55 mmol) was dissolved in 15 mL DMF, and sodium hydride (142 mg, 3.55 mmol) was added to the solution after cooling to -5°C. After stirring for 30 minutes at the same temperature, 0.7 g of compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com