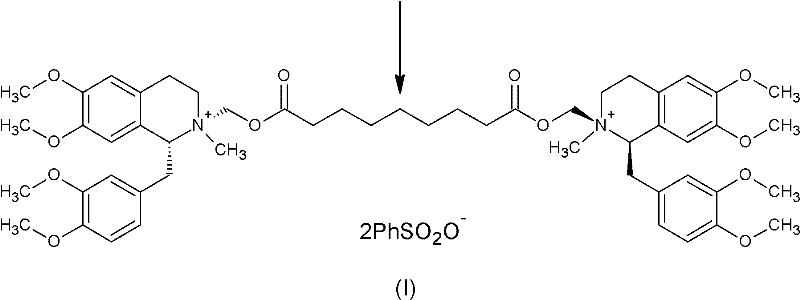
Method for preparing cisatracurium besylate
A technology of atracurium cis-benzenesulfonate and benzenesulfonic acid, which is applied in the field of preparation of atracurium cis-benzenesulfonate, can solve the problems of post-processing scheme mixing and no method for removal, etc., so as to reduce product cost, The effect of quality improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, the preparation of compound (IV)
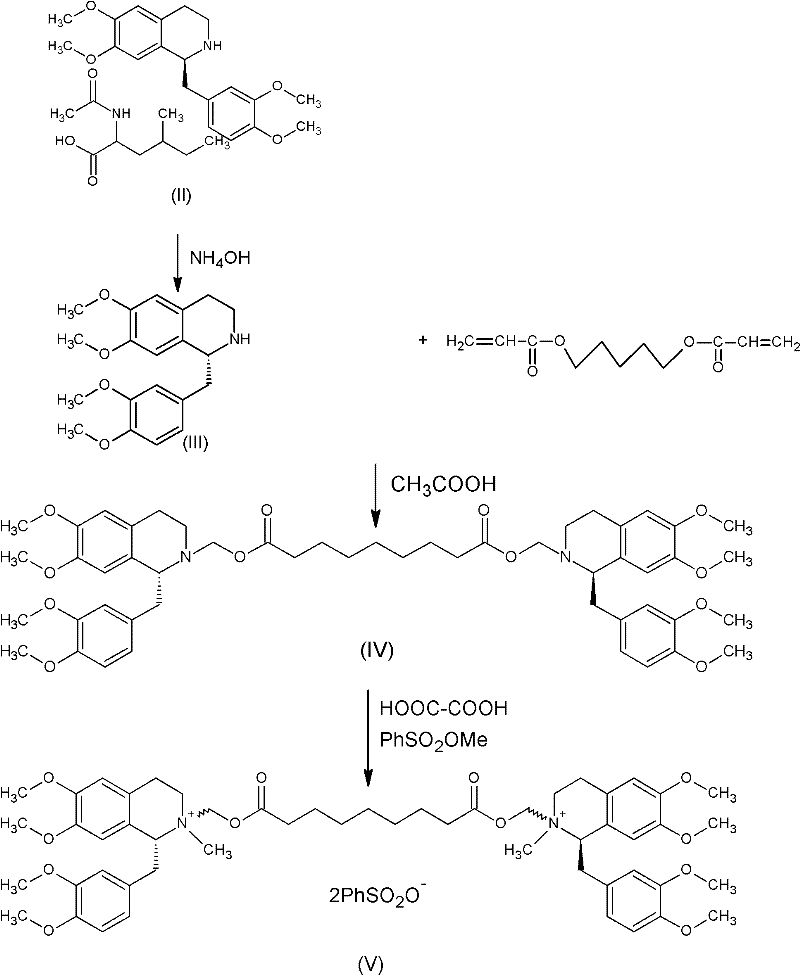
[0044] Put 700 g of R-tetrahydropapaverine-N-acetyl-L-leucine salt (II) into the reactor (the S type is less than 0.5%). Add 1.8L of water to dissolve. Adjust the pH to 7-8 with ammonia water. Extracted with 3.6L of dichloromethane, concentrated to dryness under high vacuum to obtain compound (III), added 143ml of compound 1,5-pentanediol diacrylate and 38ml of glacial acetic acid at an external temperature of 70°C for 14 hours. Reaction solution (1R, 1'R)-2,2'-(3,11-dioxo-4,10-dioxa-1,13-tridecylene)-bis[1,2,3, 4-tetrahydro-6,7-dimethoxy-1-(3,4-dimethoxy)benzyl]isoquinoline (IV) HPLC purity 92.4%. Preheated in a salt-forming reactor at 45°C 6L absolute ethanol. Dilute the reaction liquid with 400ml of dichloromethane, wash it out and add it to the salt-forming reaction tank, and then add 180g of oxalic acid. Insulate and react for 2 hours, turn off the heating, and stir at room temperature for 20 hours. Filter to g...
Embodiment 2
[0045] The refining of embodiment 2, compound (IV) oxalate:
[0046] Dissolve the crude oxalate wet product in 4L of water, extract with 4L of dichloromethane, concentrate to a small volume, add ethanol to continue concentration under reduced pressure, then add 6L of absolute ethanol, turn on cooling, stir for 20 hours, filter to obtain (1R, 1'R)-2,2'-(3,11-dioxo-4,10-dioxa-1,13-tridecylene)-bis[1,2,3,4-tetrahydro- 6,7-Dimethoxy-1-(3,4-dimethoxy)benzyl]isoquinoline oxalate wet product. Wet weight 1.5kg, HPLC99.5%.
Embodiment 3
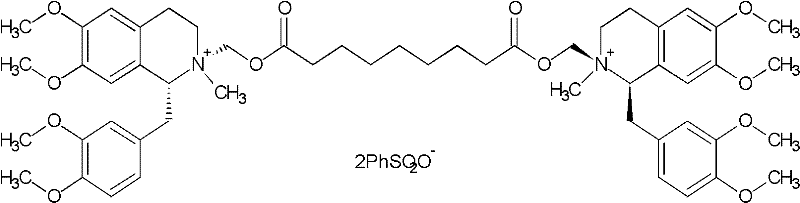
[0047] Embodiment 3, the preparation of compound (V)
[0048] Dissolve 1.5kg of wet product in 5L of water, and adjust the pH to 7-8 with ammonia water. Extract with 4 L of dichloromethane. The organic layer was dehydrated and concentrated below 60°C to an oil. Cool down to below 30°C, throw in 450ml of methyl benzenesulfonate, 700ml of acetonitrile, and 1.55g of anhydrous sodium carbonate. Reaction at an external temperature of 28-30°C for 20 hours. Diluted with 2L of dichloromethane and added dropwise to 20L of anhydrous ether. Filtration and vacuum drying for 24 hours gave 1R, 1'R-atracurium besylate 632g. HPLC purity of 3 chiral isomers and 97.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com