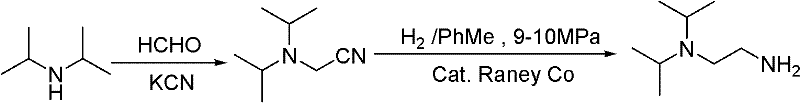Synthesis method of N,N-diisopropyl quadrol
A technology of propylethylenediamine and a synthesis method, applied in the N field, can solve the problems of large amount of waste water, polluted environment, difficult treatment and the like, and achieves the effects of cheap raw materials, simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis of diisopropylamino propionamide
[0039] Michael addition reaction: In a 500mL three-necked flask, add 71g (1mol) of acrylamide and 100mL of xylene, mechanically stir at room temperature, add 151.5g (1.5mol) of diisopropylamine dropwise, and control the rate of addition so that the reaction system does not heat up significantly. After the addition was completed, the temperature was raised to 120°C to continue the reaction for 3 hours. The reaction liquid was washed with 30 mL of saturated brine × 3 water, dried with 10 g of anhydrous sodium sulfate, and concentrated under reduced pressure to remove the solvent to obtain 86.7 g of diisopropylaminopropionamide, a colorless liquid, yield 50.4% (based on the amount of acrylamide substance, the same as in the following examples).
Embodiment 2
[0040] Embodiment 2: the synthesis of diisopropylamino propionamide
[0041]Michael addition reaction: In a 1000mL three-necked flask, add 71g (1mol) of acrylamide and 300mL of methanol, mechanically stir at room temperature to dissolve, add 151.5g (1.5mol) of diisopropylamine dropwise, and control the rate of addition so that the reaction system does not heat up significantly. After the addition, add 3.29g (0.01mol) ammonium dicyclohexyltrifluoromethanesulfonate, raise the temperature to 50°C and react for 48h, add 200mL ethyl acetate after the reaction solution is concentrated, wash the organic layer with 70mL×3 saturated saline, and wash the organic layer with 10g Dry over sodium sulfate, concentrate under reduced pressure to remove the solvent to obtain 141.8 g of diisopropylaminopropionamide, a colorless liquid, and the yield is 82.4%.
Embodiment 3
[0042] Embodiment 3: the synthesis of diisopropylamino propionamide
[0043] Michael addition reaction: In a 2000mL three-necked flask, add 71g (1mol) of acrylamide and 500mL of acetonitrile, mechanically stir at room temperature to dissolve, add 252.5g (2.5mol) of diisopropylamine dropwise, and control the rate of addition so that the reaction system does not heat up significantly. Add complete, add 36.3g (0.1mol) Zn (OTf) 2 , heated to 70°C and reacted for 56 hours, concentrated the reaction solution, added 400 mL of ethyl acetate, washed with 100 mL of saturated brine × 3, dried with 10 g of anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent to obtain 131.5 g of diisopropylaminopropionamide, light yellow Liquid, yield 76.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com