Near blue fluorescent material and preparation method thereof
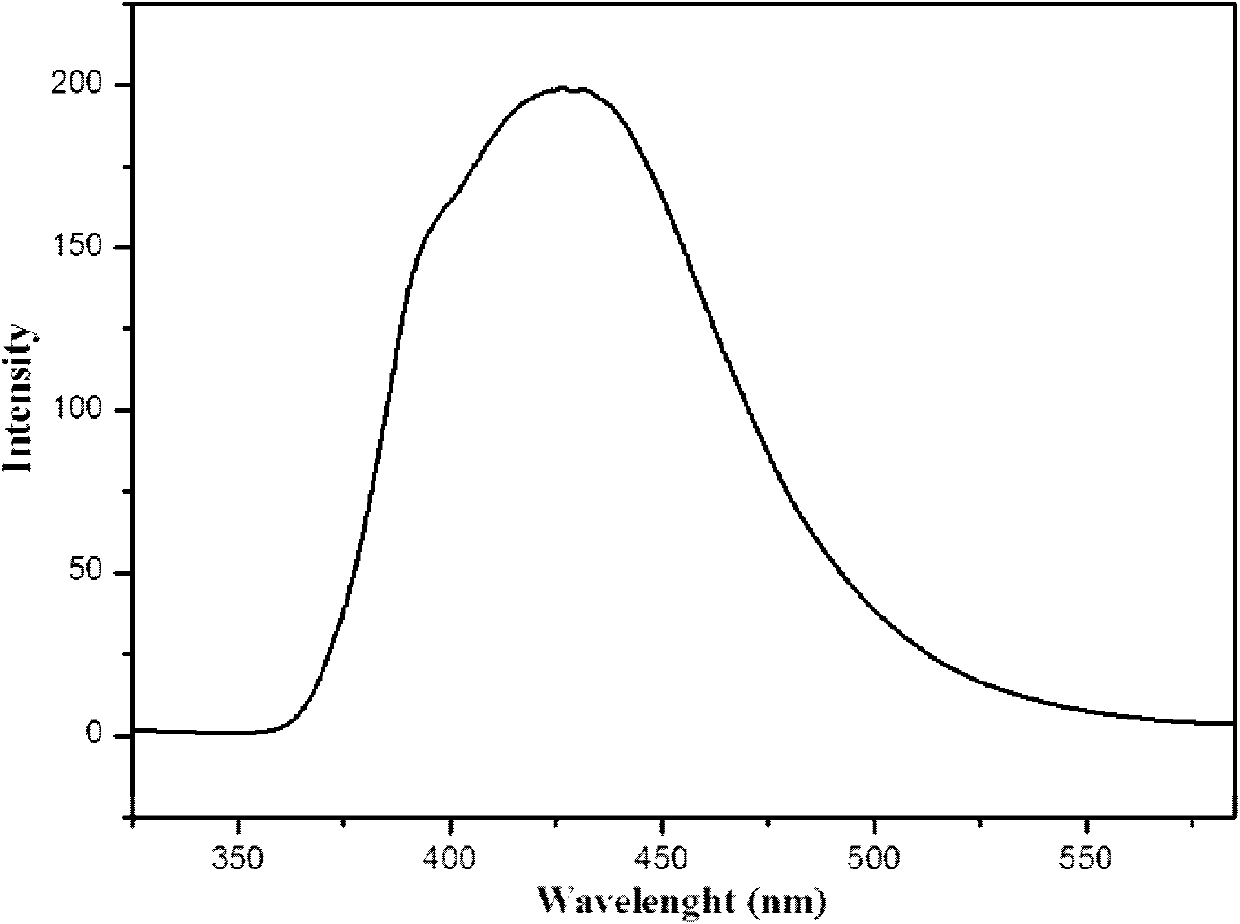
A blue fluorescence and crystal technology, applied in the field of fluorescent materials, can solve the problem of blue phosphors hindering the development of practical full-color display, and achieve good near blue light emission performance, favorable for electronic transition and energy transfer, and good photoelectric activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] To a 25 mL polytetrafluoroethylene-lined stainless steel reactor was added quinoline carboxylic acid (12.3 mg, 0.05 mmol), zinc acetate (10.5 mg, 0.05 mmol), 4,4'-bipyridine (9.8 mg, 0.05 mmol) , potassium hydroxide (2.8mg, 0.05mmol), add water 10mL, stir to make it evenly mixed.
[0020] Seal the reaction kettle and put it in an oven, heat at 60°C for 24h; then cool it down to room temperature naturally, and open the reaction kettle to obtain colorless block crystals.
Embodiment 2
[0022] To a 25 mL polytetrafluoroethylene-lined stainless steel reactor was added quinoline carboxylic acid (24.6 mg, 0.10 mmol), zinc acetate (101.0 mg, 0.50 mmol), 4,4'-bipyridine (99.0 mg, 0.50 mmol) , potassium hydroxide (5.6mg, 0.10mmol), add water 15mL, stir to make it evenly mixed.
[0023] Seal the reaction kettle and place it in an oven, heat at 100°C for 48h; then cool down to room temperature naturally, and open the reaction kettle to obtain colorless block crystals.
Embodiment 3
[0025] To a 25 mL polytetrafluoroethylene-lined stainless steel reactor was added quinoline carboxylic acid (36.9 mg, 0.15 mmol), zinc acetate (31.5 mg, 0.15 mmol), 4,4'-bipyridine (19.8 mg, 0.10 mmol) , potassium hydroxide (11.2mg, 0.20mmol), add 20mL of water, stir to make it evenly mixed.
[0026] Seal the reaction kettle and place it in an oven, heat at 120°C for 72h; then cool it down to room temperature naturally, and open the reaction kettle to obtain colorless blocky crystals.
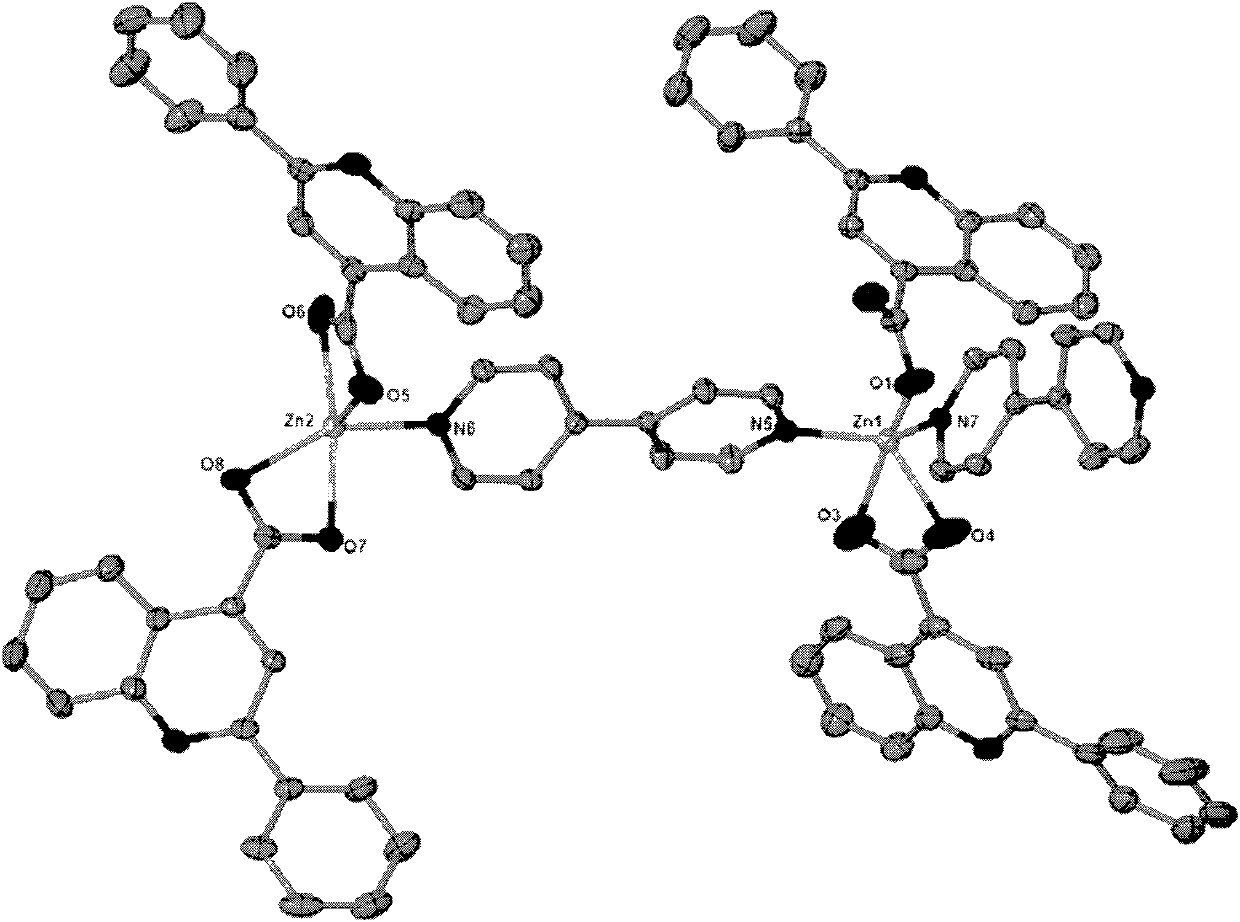
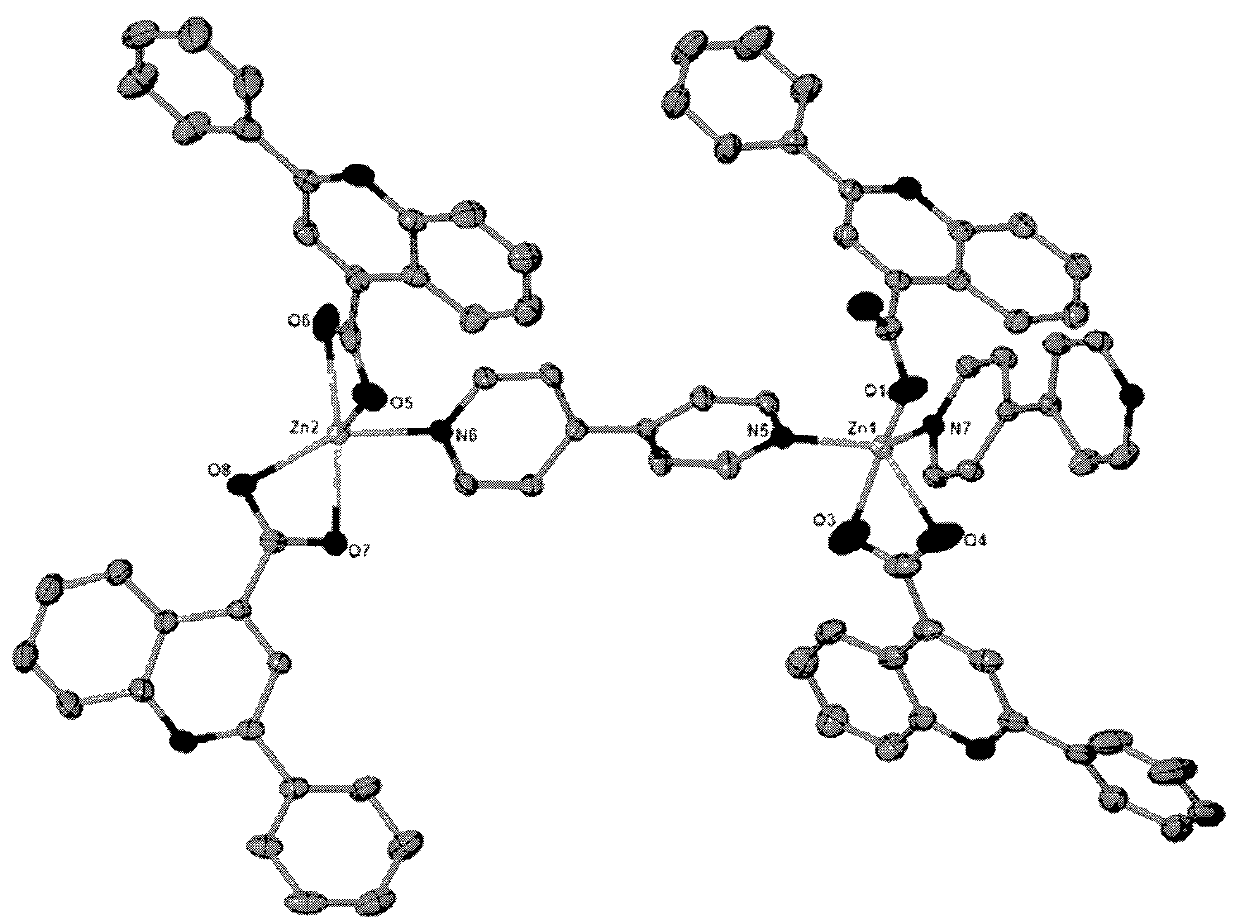
[0027] The obtained colorless block crystals are rinsed with distilled water and air-dried naturally; after determination, the molecular formula of the zinc quinoline carboxylic acid crystals is C 84 h 56 N 8 o 8 Zn 2 , the crystal system is triclinic, the space group is P-1, and the unit cell parameters α=91.357°, γ=106.573°, β=101.953°, zinc ions have two coordination modes: one is tetragonal pyramid coordination mode, and the two nitrogen coordination atoms come from two differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com