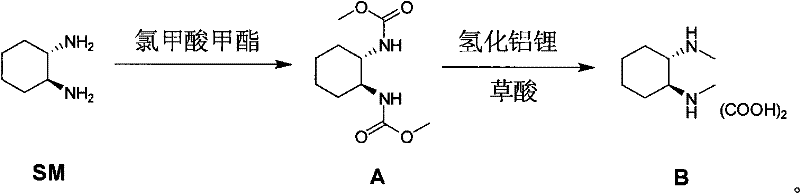
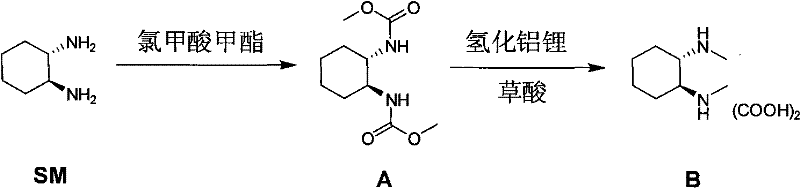
Method for synthesizing (1R,2R)-(-)-N,N-dimethyl-1,2-cyclohexanediamine oxalate
A technology for the synthesis of cyclohexanediamine oxalate, which is applied in the field of -N, can solve the problems of limited preparation process, low preparation cost, and inability to scale production, and achieve cheap raw materials, short production time, and optimized post-processing The effect of craft
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] (1R,2R)-(-)-N,N-Dimethyl-1,2-cyclohexanediamine oxalate
[0031] The first step (amidation): Add 100 g of the raw material to 750 mL of dichloromethane and 500 mL of 13% sodium hydroxide solution at room temperature, then cool it down to 0-10°C, add 75 g of methyl chloroformate dropwise, and react at room temperature for 1-4 hours. Add 100mL of water and 500mL of dichloromethane in turn, static layering, wash the organic layer once with 600mL of water, concentrate the organic layer to 300-400mL, add 1L of n-heptane, concentrate to 300-400mL, then add 1L of n-heptane , concentrated to 300-400 mL, the concentrated solution was stirred at room temperature for 10-12 h, filtered and rinsed once with 100-200 mL of n-heptane, and the solid intermediate A (83 g, 95%) was vacuum-dried.
[0032] The second step (reduction): Add 68g of lithium aluminum tetrahydrogen in batches to 600mL of anhydrous tetrahydrofuran at 0~10°C, stir for 10min, then slowly add a solution of 83g of int...
example 2
[0034] (1R,2R)-(-)-N,N-Dimethyl-1,2-cyclohexanediamine oxalate
[0035] The first step (amidation): Add 100g of raw material to 750mL of dichloromethane and 500mL of 13% sodium hydroxide solution at room temperature, then cool it down to 0-10°C, add 75g of methyl chloroformate dropwise, and react at room temperature for 1-4h , add 100mL of water and 500mL of dichloromethane in turn, static layering, wash the organic layer once with 600mL of water, concentrate the organic layer to 300-400mL, add 1L of n-heptane, concentrate to 300-400mL, then add 1L of n-heptane Alkanes, concentrated to 300-400mL, the concentrated solution was stirred at room temperature for 10-12h, filtered and rinsed once with 100-200mL of n-heptane, and the solid intermediate A (83g, 95%) was vacuum-dried.
[0036] The second step (reduction): Add 82g of lithium aluminum tetrahydrogen in batches to 600mL of anhydrous tetrahydrofuran at 0~10°C, stir for 10min, then slowly add a solution of 83g of intermediate...
example 3
[0038] (1R,2R)-(-)-N,N-Dimethyl-1,2-cyclohexanediamine oxalate
[0039]The first step (amidation): Add 750mL of dichloromethane and 500mL of 13% sodium hydroxide solution to the raw material at room temperature, then cool it down to 0-10°C, add 75g of methyl chloroformate dropwise, react at room temperature for 1-4 hours, and then Add 100mL of water and 500mL of dichloromethane, keep the layers, wash the organic layer once with 600mL of water, concentrate the organic layer to 300-400mL, add 1L of n-heptane, concentrate to 300-400mL, then add 1L of n-heptane, Concentrated to 300-400 mL, the concentrated solution was stirred at room temperature for 10-12 h, filtered and rinsed with 100-200 mL of n-heptane once, and solid intermediate A (83 g, 95%) was vacuum-dried.
[0040] The second step (reduction): Add 68g of lithium aluminum tetrahydrogen in batches to 600mL of anhydrous tetrahydrofuran at 0~10°C, stir for 10min, then slowly add a solution of 83g of intermediate A dissolved...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com