Medicament for treating and/or preventing viral infection
A technology of drugs and viruses, applied in the field of new protein drugs and adjuvants for the treatment and/or prevention of viral infections, and drugs for the treatment and/or prevention of viral infections, which can solve the problems of weak immunogenicity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1. Cloning and expression of interleukin 17A
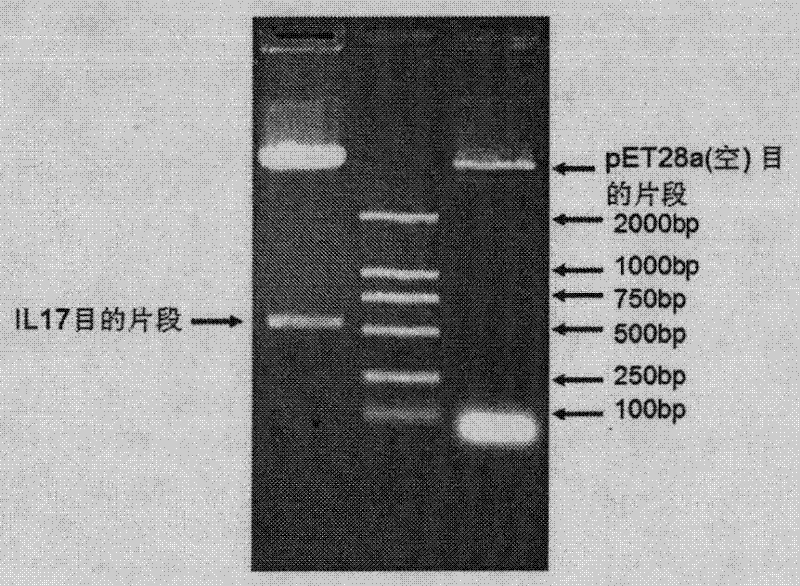
[0047] Since the prokaryotic expression system cannot process and cut the interleukin 17A signal peptide, when designing primers, clone and express it from the downstream of the signal peptide, so the following experiments all use IL-17A without signal peptide sequence.
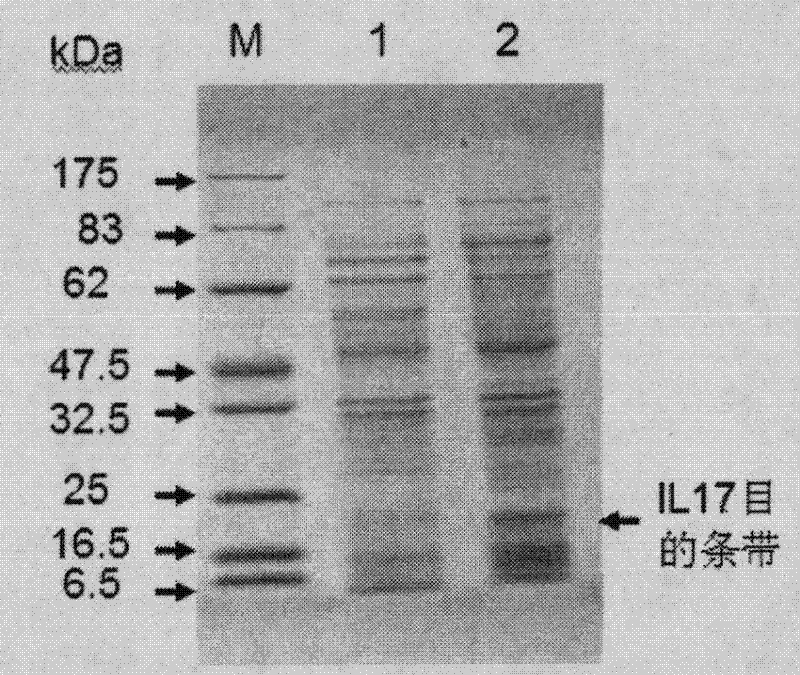
[0048] The mouse IL-17A expression plasmid is a reverse transcription of RNA extracted from the mouse spleen, and the IL-17 gene is cloned from cDNA. The PCR product was recovered after electrophoresis, and ligated with pMD18-T vector to construct pMD18-T-IL-17, which was then digested with restriction enzymes BamH I and Xba I. The pMD18-T-IL-17 with the correct restriction analysis was further sequenced, and the sequencing result was aligned with the IL-17A sequence number NM_010552 on GeneBank, which was completely consistent. The human IL-17A cDNA clone was obtained by artificial synthesis using the sequence number NM_002190 on GeneBank. The artific...
Embodiment 2
[0052] Example 2. Detection of interleukin 17A biological activity
[0053] In order to prove that the expressed IL-17A is biologically active, we use IL-17 as a pro-inflammatory factor to stimulate innate immune cells to produce IL-6 and TNF-α, so we put IL-17 at different concentrations ( 50ng / ml and 100ng / ml) treat RAW264.7 cells, 48 hours after stimulation, detect the secretion level of IL-6, such as image 3 As shown, the expressed IL-17A can stimulate RAW264.7 cells to secrete I1-6, and this stimulation is dose-dependent.
Embodiment 3
[0054] Example 3. Experiments of Interleukin 17A Protecting Animals Against Influenza Virus Attack
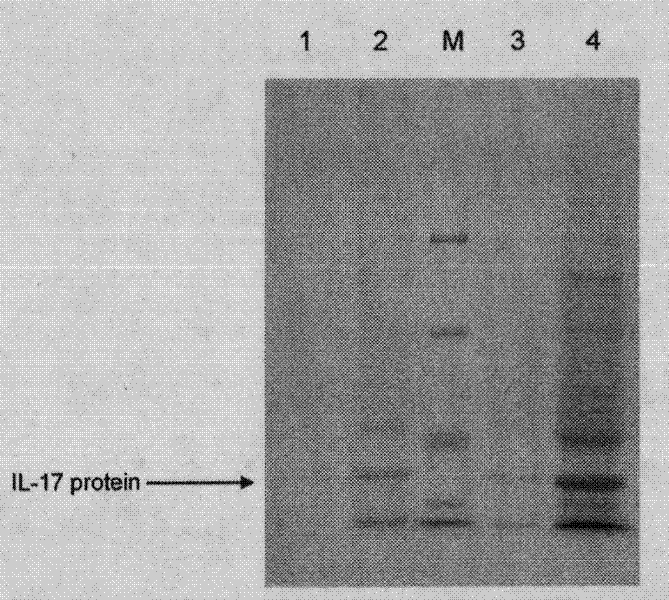
[0055] Interleukin 17A was cloned in the pET28a plasmid in the laboratory, and purified by E. coli expression. 100ug of interleukin 17A was dissolved in 10ml of 10mM pH 7.0 PBS buffer to obtain a 10ug / ml solution. Female Balb / c mice aged 6-8 weeks were divided into 3 groups, each with 15 mice, and the other group was interleukin 17A knockout Balb / c mice (IL-17KO). Each group of mice were injected with 0ul, 10ul or 50ul of the above-mentioned interleukin 17A, repeated at intervals of 6 days, a total of 2-4 times. Seven days later, each mouse was inoculated with H5N1 influenza virus with LD50=5. On the 6th day, the lungs of 3 mice from each group were extracted for RNA extraction and real-time fluorescent quantitative RT-PCR to analyze the viral load ( Figure 4 ), observe the death of the remaining animals every day ( Figure 5 ). The result is figure 1 As shown, the virus RNA co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com