Mycobacterium tuberculosis TB10.4-F1 fusion protein vaccine and preparation method thereof
A TB10.4-F1, TB10.4 technology, applied in chemical instruments and methods, medical preparations containing active ingredients, hybrid peptides, etc., can solve hospitalization and increased mortality, unacceptable virulence, enhanced To achieve the effects of easy cultivation and fermentation, high yield of target protein, and improved expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Obtaining of TB10.4(N / X), TB10.4(N / B), F1(B / X) genes.
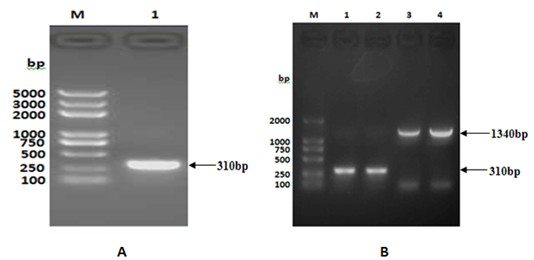
[0037] Using the whole genome of Mycobacterium tuberculosis standard strain H37Rv as a template, TaqPlus DNA polymerase (from TIANGEN, J8422) was used to amplify the TB10.4 gene. The primers used were as follows: THP1: 5'-CATATGTCGCAAATCATGTACAACTACCC -3', THP2 : 5'-GCGCCGGATCCGGCGCCGCCGCCCCATTTGGCGGCTTCGGCCGTGTC-3', TBP2: 5'-CTCGAGTTAGTGGTGGTGGTGGTGGTGGCCGCCCCATTTGGCGGCTTC-3'. Among them, THP1 and THP2 are used to amplify the TB10.4(N / B) gene, and introduce Nde Ⅰ (from TAKALA company, CK8852A), Bam H Ⅰ (from TAKALA company, CK8701A) two restriction sites and linker sequence are used to construct the fusion gene TB10.4-F1; THP1 and TBP2 are used to amplify the TB10.4(N / X) gene and introduce Nde I and xho Ⅰ (from TAKALA company, CK1701A) two restriction sites and 6×His tag, used to express TB10.4 protein alone; PCR amplification conditions are: 95°C 10min; 95°C 30s, 52°C 30s, 72°C 20s (30 cycles);...
Embodiment 2
[0039] Example 2: Construction of recombinant subcloning vectors TB10.4(N / X) / pMD18T, TB10.4(N / B) / pMD18T and F1(B / X) / pMD18T.
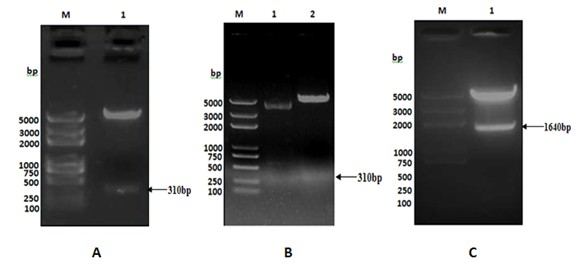
[0040] After purifying TB10.4(N / X), TB10.4(N / B) and F1(B / X) genes using the gel recovery kit from Biotech Corporation (the following gel recovery and purification are all performed according to the instructions), each 50ng were ligated with 50ng of pMD18T (from TAKALA, CK5001BA) with 1 μl of T4 DNA ligase (from TAKALA, CK5021B) overnight at 16°C, and the ligated products were transformed by heat shock method with CaCl 2 Prepared E. coli DH5α, Coated Amp + -LB plate, cultured at 37°C for 16 hours, picked several single colonies to inoculate Amp + - LB culture medium, cultured with shaking at 37°C for 12 hours, and then extracting the plasmid. use Nde I and xho Ⅰ After double digestion of recombinant vector TB10.4(N / X) / pMD18T (recombinant plasmid: 700ng; enzyme: 1μl each; 20μl total system, placed at 37℃ for 3h), the target fragment with a size o...
Embodiment 3
[0041] Example 3: Construction of recombinant expression vectors TB10.4 / pET28a, TB10.4(N / B) / pET30a, TB10.4-F1 / pET30a.
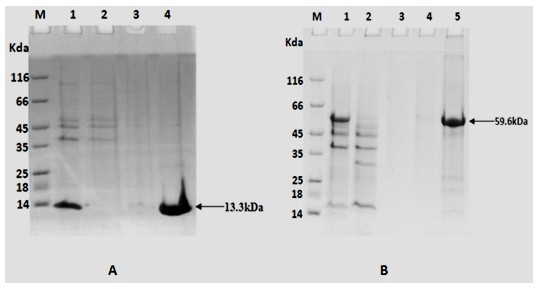
[0042]Construction of the recombinant expression vector TB10.4 / pET28a: use the correct TB10.4(N / X) / pMD18T and pET28a plasmids Nde I and xho Ⅰ Digest at 37°C for 3 hours, take the gel to recover 50ng of the purified TB10.4(N / X) fragment and 150ng of the purified expression vector pET28a double digestion product, and use 1μl of T4 DNA ligase to ligate overnight at 16°C. CaCl for transformation by shock method 2 Prepared E. coli DH5α, coated Kan + -LB plate, cultivated at 37°C for 16 hours, picked several single colonies to inoculate Kan + - LB culture medium, cultured with shaking at 37°C for 12 hours, and then extracting the plasmid. use Nde I and xho Ⅰ After double enzyme digestion (2 μg of recombinant plasmid; 2 μl of each enzyme; 50 μl of the total system at 37°C for 5 hours), the target fragment with a size of 310 bp and its corresponding vect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com