Peptide formulations and uses thereof
A technology for preparations and water-based preparations, applied in the field of preventive and therapeutic drugs, can solve the problems of application limitation, influence, and stability reduction of therapeutic methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 (comparative example)
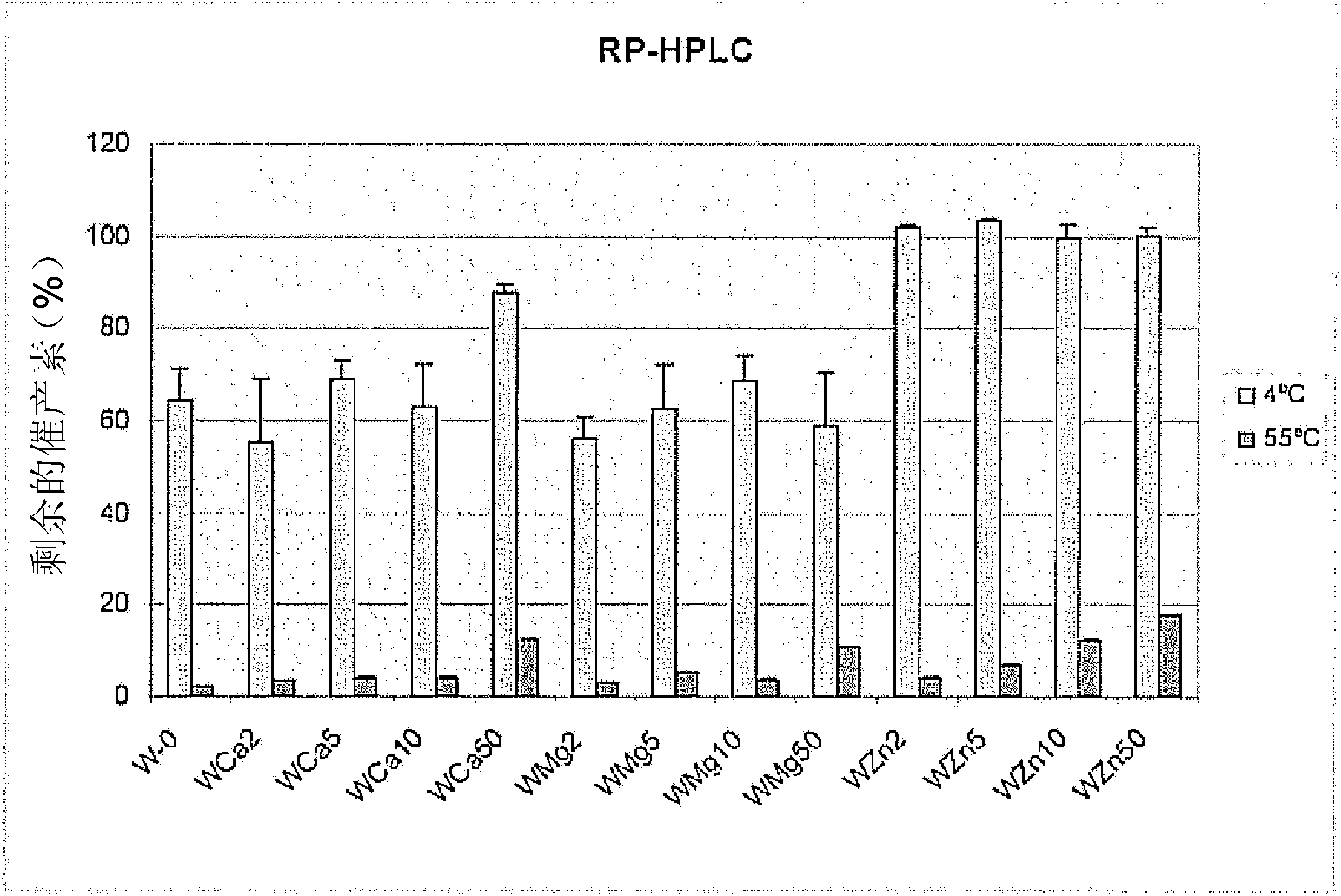
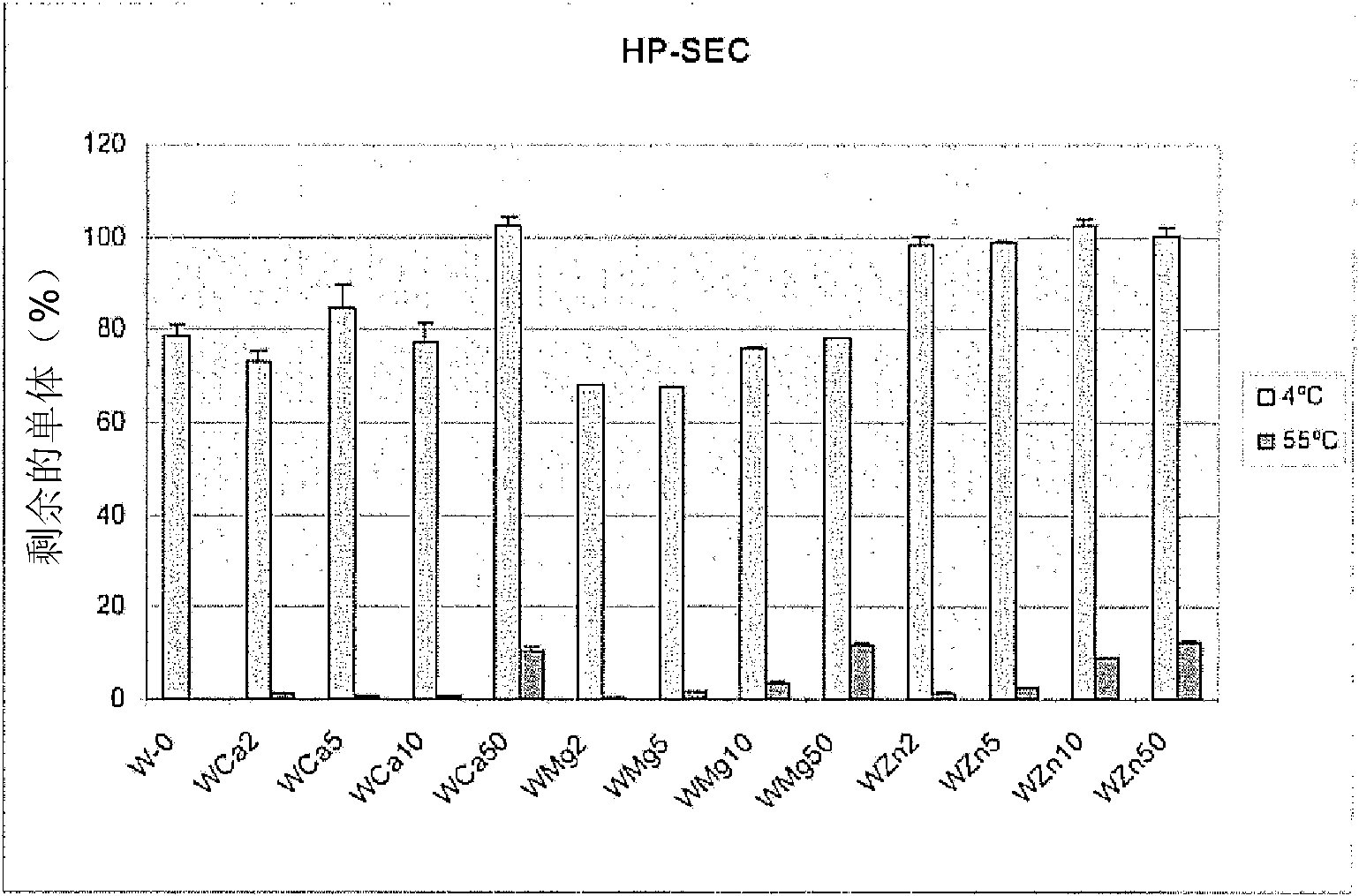
[0062] This comparative example demonstrates the oxytocin-stabilizing effect of divalent metal ions. Will be used as metal chloride salt (MCl 2 ) of divalent metal ions (Ca 2+ , Mg 2+ and Zn 2+ ) was added to an unbuffered formulation including oxytocin and purified water (W). Divalent metal ions were used at concentrations of 0 mM, 2 mM, 5 mM, 10 mM and 50 mM. Formulation samples were then stored at pH 4.5 at 4°C (reference control) or 55°C (test) for 4 weeks. Thereafter, all samples were stored under cooling conditions (2-8 °C) before RP-HPLC and HP-SEC analysis. Effects on stability were determined by measuring oxytocin recovery and percent oxytocin monomer. Figure 1A and 1B The results are shown in .
[0063] Figure 1A Oxytocin recovery after storage at 55° C. for 4 weeks in unbuffered pure water (W) in the absence or presence of divalent metal ions is shown. in ca 2+ or Zn 2+ Oxytocin recovery increased at 4°C in th...
Embodiment 2
[0064] Embodiment 2 (comparative example)
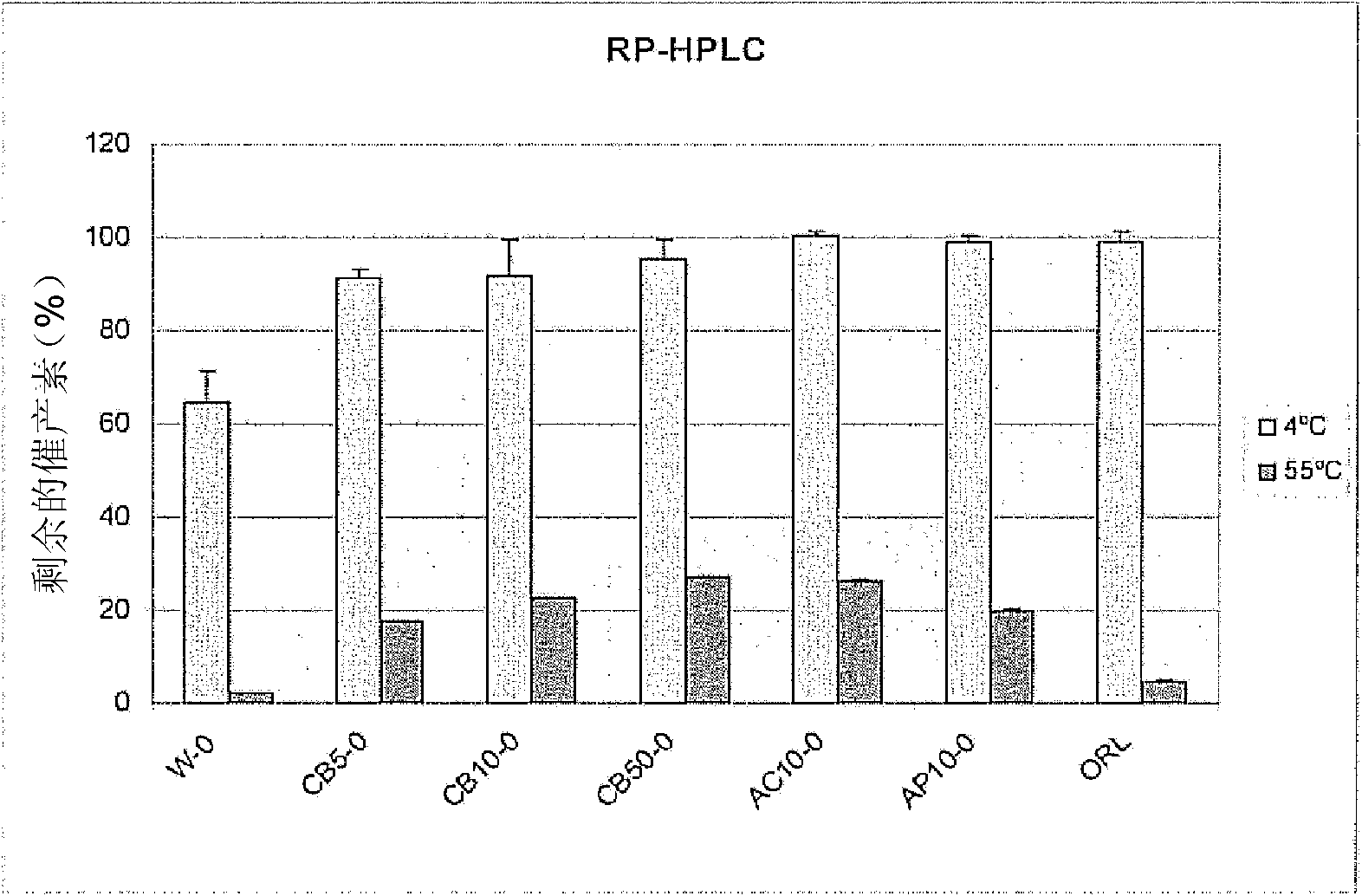
[0065] Citrate buffer (CB) at a concentration of 0 mM, 5 mM, 10 mM or 50 mM, acetate buffer (AC) at a concentration of 10 mM, aspartate buffer (AP) at a concentration of 10 mM or Ringer Lactate buffer (ORL) was added to the formulation including oxytocin in purified water (W). The Ringer's lactate used was Baxter Viavlo 500 mL WE2323 Ringer's lactate solution for intravenous infusion. Batch number 09B04E1P, expiration date (exp.date) 2011. The pH of the preparation was 6.4.
[0066] Composition per 100mL:
[0067]
[0068] All formulations were free of divalent metal ions (except ORL) and had a final concentration of oxytocin of 0.1 mg / ml and a pH of 4.5 (CB, AC and AP) or 6.4 (ORL). Samples of each formulation were stored at 4°C (control) or 55°C (test) for 4 weeks. Thereafter, all samples were stored under cooling (2-8° C.) prior to RP-HPLC and HP-SEC analysis. The effect on stability was determined by measuring the recove...
Embodiment 3
[0070] Embodiment 3 (comparative example)
[0071] The thermal stability of oxytocin in the presence of a combination of buffers and monovalent metal ions was investigated. Use citrate buffer (CB), acetate buffer (AC) or aspartate buffer (AP) at a concentration of 10 mM. Addition of the monovalent metal ion Na in its chloride salt form (NaCl and KCl) + and K + , using final concentrations of 10 mM and 20 mM. All formulations had a final concentration of oxytocin of 0.1 mg / ml and a pH of 4.5. Samples of each formulation were stored at 4°C (control) or 55°C (test) for 4 weeks. Thereafter, all samples were stored under cooling (2-8° C.) prior to RP-HPLC and HP-SEC analysis. The effect on stability was determined by measuring the recovery of oxytocin and the percentage of oxytocin monomer. Figure 3A and 3B The results are shown in .
[0072] exist Figure 3A In , a minor thermostability effect of oxytocin stored at 55°C was observed. Despite the presence of buffer alone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com