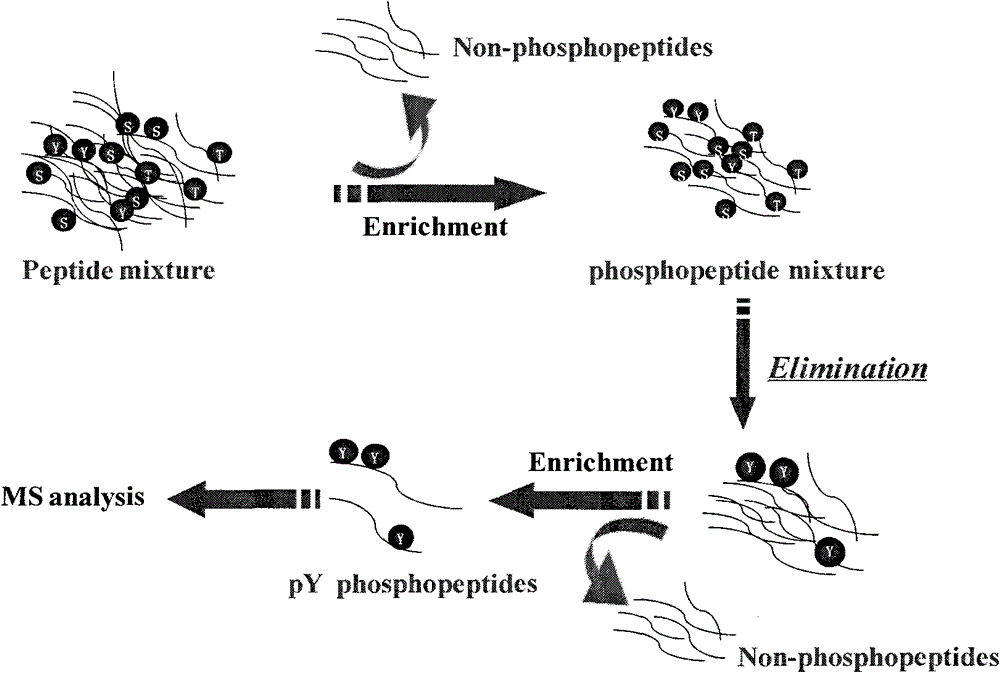
Method for purifying tyrosine phosphopeptide
A technology of tyrosine phosphorylation and tyrosine phosphopeptide, which is applied in the purification field of tyrosine phosphorylated peptide, can solve the problems of harsh solvent and temperature requirements, limited reagent price, difficult protein identification, etc., and achieves high sensitivity, Highly selective, highly specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] Preparation of sample solution: 1 mg each of α-casein and β-casein were dissolved in 1 mL of 50 mM ammonium bicarbonate solution (pH 8.0), according to the mass ratio of trypsin to 1:40 (w / w) Trypsin was added for enzymolysis reaction, the reaction time was 16 hours, and the enzymolysis temperature was controlled at 37°C. The obtained proteolysis solution was stored in a -30°C refrigerator for future use. Dissolve 1mg of protein bovine serum albumin in 1mL of reducing solution ((pH 8.0, 8M urea, 50mM ammonium bicarbonate solution), leave it at room temperature for 4h, then add DTT solution to the centrifuge tube containing inactivated protein , placed in a water bath at 37°C for 2 hours. Take the solution out of the water bath, add IAA solution to each tube of solution, and let it stand in the dark at room temperature for 30 minutes. After the above treatment, the protein is completely denatured, the disulfide bond is opened, and the sulfhydryl group is blocked. Then t...
Embodiment 1
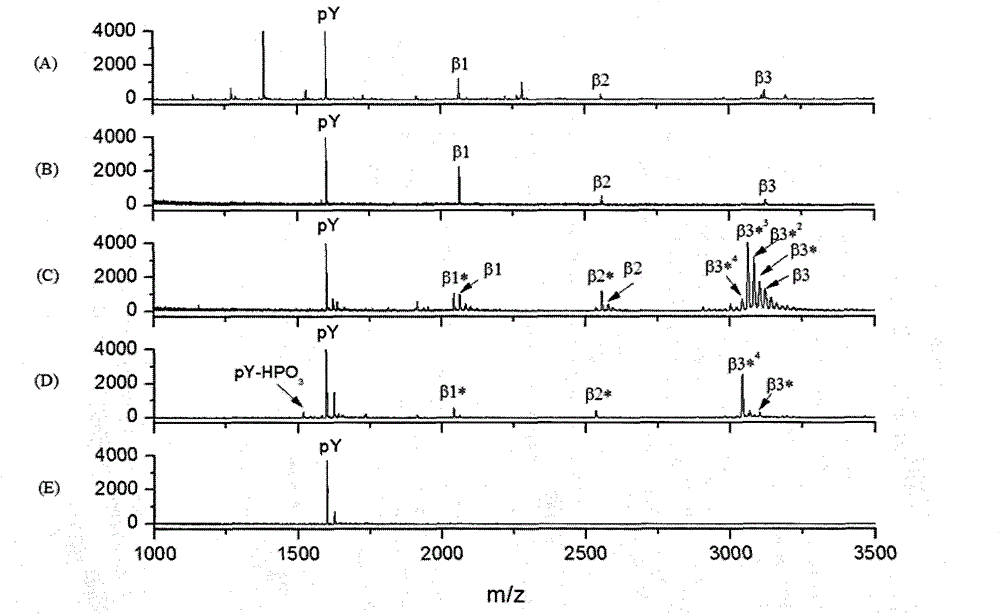
[0033] Example 1. β-elimination and Michael nucleophilic addition reaction method for purification of tyrosine phosphopeptides
[0034] (1) The enzymatic hydrolysis product of the standard phosphorylated protein β-casein and pY are mixed at a molar ratio of 1 pmol: 1 pmol.
[0035] (2) Use Ti 4+ -IMAC monodisperse microspheres enrich and purify phosphopeptides. Disperse the above-prepared samples in 50% ACN, 6% TFA, and 200 mM NaCl on Ti 4+ - The suspension of IMAC monodisperse microspheres (10mg / mL) was mixed according to the mass ratio of sample and microspheres at 1:10, incubated with shaking at room temperature for 30min, then centrifuged at 30000g at high speed, discarded the supernatant, and added an equal volume of Aqueous solution containing 200mM NaCl, 50% ACN, 6% TFA, shaking to clean Ti 4+ -IMAC monodisperse microspheres for 15min, then centrifuge at 30,000g at high speed, discard the supernatant, then add twice the volume of an aqueous solution containing 0.1% T...
Embodiment 2
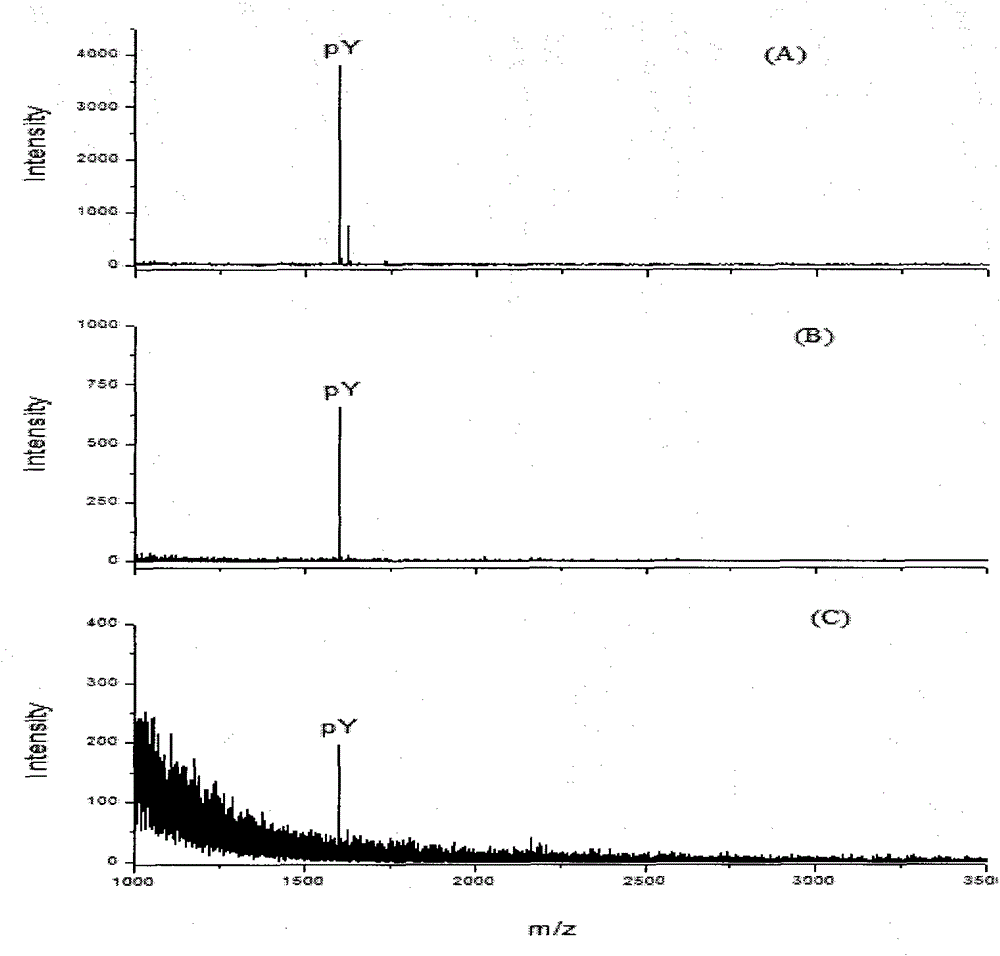
[0042] Example 2. The difference from Example 1 is that in the complex sample system, the moles of pY, α-casein, β-casein and BSA are 1:10:10:10 and 1:100:100:100. Depend on Figure 4 It can be seen that in the complex sample system, the molar ratio of pY, α-casein, β-casein and BSA is 1:10:10:10 and 1:100:100:100, the untreated sample is very complex, but after β-elimination The sample treated with the Michael nucleophilic addition reaction method left only pY, and it was very clean with a very good signal-to-noise ratio. This shows that the method of β-elimination / Michael nucleophilic addition reaction has very high specificity for purifying P-Tyr phosphopeptide.
[0043] purification
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com