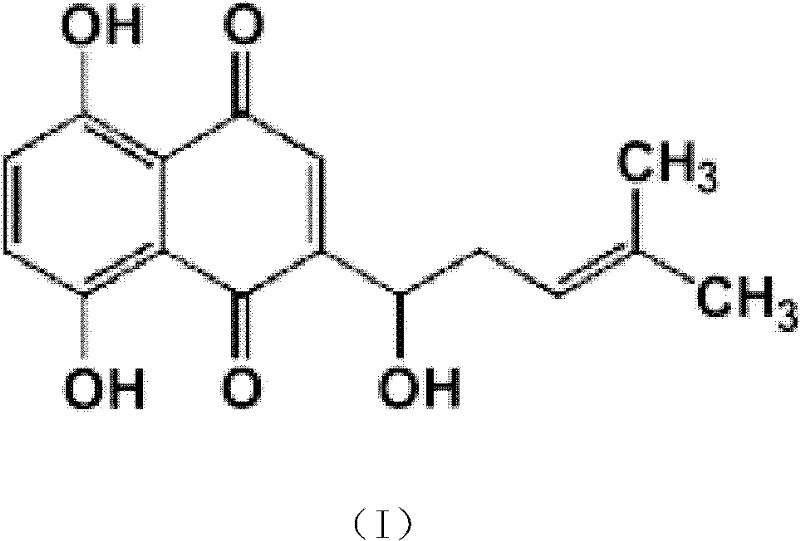
Use of shikonin as antifungal medicine synergist
An antifungal drug, shikonin technology, applied in the field of medicine to reduce toxic side effects and treat fungal infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Example 1: Effects of combined use of shikonin and fluconazole on different clinical fungal strains.
[0011] Materials and methods
[0012] 1. Test drug:
[0013] Shikonin: National Institute for the Control of Pharmaceutical and Biological Products (the same below).
[0014] Fluconazole: Pfizer Pharmaceutical Co., Ltd. (the same below).
[0015] Dimethyl sulfoxide: China Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0016] The concentration of shikonin was 8 mg / ml, the concentration of fluconazole was 2 mg / ml, and the tested drugs were stored at -20°C. Before the experiment, the drug stock solution was taken out and placed in a 35°C incubator to melt, fully mixed, and the pharmacodynamic test was carried out respectively.
[0017] 2. Strains:
[0018] The clinical strains of Candida albicans, Candida krusei and Microsporum lanoides were provided by the fungal laboratory of Shanghai Changhai Hospital, and were identified by morphology and biochemistr...
Embodiment 2
[0046] Example 2: The effect of shikonin combined with fluconazole on different clinical and laboratory induced drug-resistant strains
[0047] Materials and methods
[0048] 1. Test drug:
[0049] The sources of shikonin and fluconazole are the same as above.
[0050] Dimethyl sulfoxide: China Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0051] The concentration of shikonin was 8 mg / ml, the concentration of fluconazole was 2 mg / ml, and the tested drugs were stored at -20°C. Before the experiment, the drug stock solution was taken out and placed in a 35°C incubator to melt, fully mixed, and the pharmacodynamic test was carried out respectively.
[0052] 2. Strains:
[0053] Clinical drug-resistant strain: Candida albicans, provided by the Fungi Department of Shanghai Changhai Hospital, confirmed by morphological and biochemical identification.
[0054] Drug-resistant strains induced in the laboratory: Candida albicans (SC5314-R, Y01-R) are drug-resistant stra...
Embodiment 3
[0062] Embodiment 3: Combination of shikonin and miconazole, ketoconazole
[0063] Materials and methods
[0064] 1. Test drug:
[0065] Shikonin: National Institute for the Control of Pharmaceutical and Biological Products.
[0066] Fluconazole: Pfizer Pharmaceuticals Ltd.
[0067] Ketoconazole: National Institute for the Control of Pharmaceutical and Biological Products.
[0068] Miconazole: National Institute for the Control of Pharmaceutical and Biological Products.
[0069] Dimethyl sulfoxide: China Pharmaceutical (Group) Shanghai Chemical Reagent Company.
[0070] The concentration of shikonin was 8 mg / ml, the concentration of miconazole and ketoconazole was 6.4 mg / ml, and the tested drugs were stored at -20°C. Before the experiment, the drug stock solution was taken out and placed in a 35°C incubator to melt, fully mixed, and the pharmacodynamic test was carried out respectively.
[0071] Other experimental steps and methods are the same as in Example 1.
[0072]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com