Process for preparing Cinacalcet hydrochloride tablets or capsules
A kind of technology of cinacalcet hydrochloride and capsule, applied in the field of preparation of pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of Cinacalcet Hydrochloride 30mg, 60mg, 90mg Sustained Release Tablets
[0026] prescription
quantity
content%
33mg
18.33%
6000
15mg
8.33%
20mg
11.11%
70mg
38.89%
Crospovidone
13mg
7.22%
partially pregelatinized starch
27mg
15.00%
2mg
1.12%
Total
180g
100%
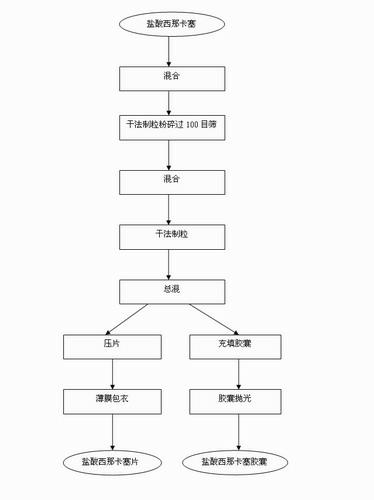
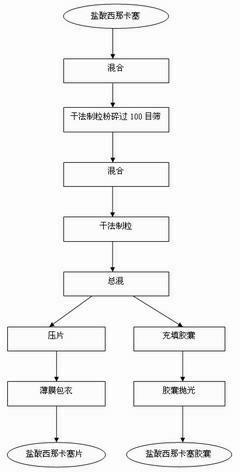
[0027] 1) Cinacalcet hydrochloride was crushed through a 100-mesh sieve, and mannitol, microcrystalline cellulose, crospovidone, and partially pregelatinized starch were passed through a 60-mesh sieve.
[0028] 2) Add to the mixer and mix for 15 minutes,
[0029] 3) Dry granulation in the dry granulator, and the granules pass through a 20-mesh sieve.
[0030] 4) Mix the granules of operation 3) with magnesium stearate evenly.
[0031] 5 tablets
[0032] 30mg 180mg / tablet 100 tablets
[0033...
Embodiment 2
[0046] Preparation of Cinacalcet Hydrochloride 30mg, 60mg, 90mg Sustained Release Tablets
[0047] prescription
quantity
content%
Cinacalcet hydrochloride
33mg
16.50%
polyethylene glycol 6000
15mg
7.50%
25mg
12.50%
75mg
37.50%
Crospovidone
18mg
9.00%
partially pregelatinized starch
32mg
16.00%
2mg
1.00%
Total
200mg
100%
[0048] 1) Cinacalcet hydrochloride was crushed through a 100-mesh sieve, and mannitol, microcrystalline cellulose, crospovidone, and partially pregelatinized starch were passed through a 60-mesh sieve.
[0049] 2) Add to the mixer and mix for 15 minutes,
[0050] 3) Dry granulation in the dry granulator, and the granules pass through a 20-mesh sieve.
[0051] 4) Mix the granules of operation 3) with magnesium stearate evenly.
[0052] 5 tablets
[0053] 30mg 200mg / tabl...
Embodiment 3
[0067] Preparation of Cinacalcet Hydrochloride 30mg, 60mg, 90mg Sustained Release Tablets
[0068] prescription
quantity
content%
Cinacalcet hydrochloride
33mg
15.00%
polyethylene glycol 6000
15mg
6.82%
30mg
13.64%
80mg
36.36%
Crospovidone
23mg
10.45%
partially pregelatinized starch
37mg
16.82%
Magnesium stearate
2mg
0.91%
Total
220mg
100%
[0069] 1) Cinacalcet hydrochloride was crushed through a 100-mesh sieve, and mannitol, microcrystalline cellulose, crospovidone, and partially pregelatinized starch were passed through a 60-mesh sieve.
[0070] 2) Add to the mixer and mix for 15 minutes,
[0071] 3) Dry granulation in the dry granulator, and the granules pass through a 20-mesh sieve.
[0072] 4) Mix the granules of operation 3) with magnesium stearate evenly.
[0073] 5 tablets
[0074] 30mg 220mg / tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com