Bilobalide B hydrolyzed derivatives and application thereof
A technology of ginkgolide and its derivatives, which is applied in the field of medicine, can solve the problem of no 10-O-(dimethylaminoethyl) ginkgolide, etc., and achieve the effect of ensuring safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Separation and Preparation of Ginkgolide B Hydrolyzed Derivatives
[0019] Take an appropriate amount of 10-O-(dimethylaminoethyl) ginkgolide B methanesulfonate, add methanol to dissolve, separate with TLC, and use CHCl 3 / CH 3 OH (6 / 1) was developed repeatedly to obtain two spots with lower Rf value than the raw material, which were separated on a preparative plate, and the two products obtained were further separated by HPLC preparative column to obtain two compounds (A and B).
Embodiment 2
[0020] Example 2 Synthesis of Ginkgolide B Hydrolyzed Derivatives
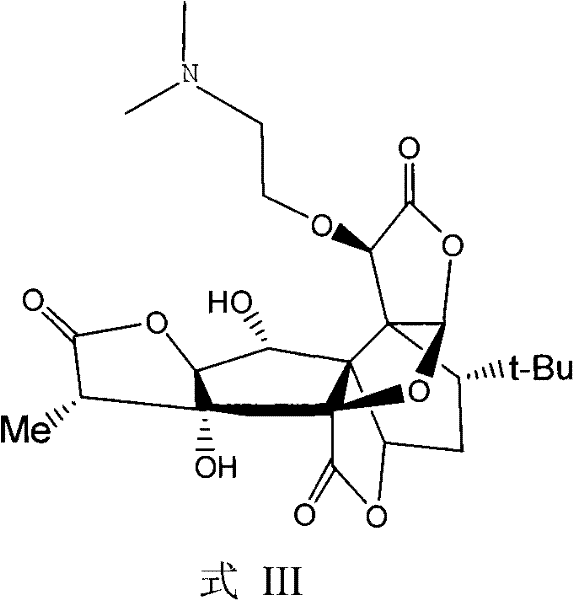
[0021] Take about 5.0 g of 10-O-(dimethylaminoethyl) ginkgolide B methanesulfonate, dissolve it in water, add about 1.0 g of sodium carbonate powder, stir at 50°C for 6 hours, filter, and wash the filtrate with ethyl acetate for two Once, the aqueous layer was evaporated to dryness to obtain a white powder, which was separated by column chromatography, CHCl 3 / CH 3 OH (6 / 1) was eluted, and the desired eluent was collected, evaporated to dryness, and dried to obtain two compounds (C and D).
Embodiment 3
[0022] Example 3 Structure Confirmation
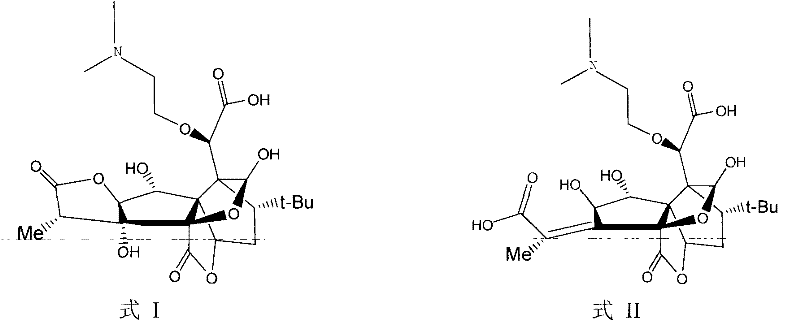
[0023] After analysis and identification, the compound C obtained in Example 2 is the same as the compound A obtained in Example 1, and the molecular structure is shown in Formula 1. MS(m / z): 514[M+H] +1 ; 1 H-NMR (CDCl 3 ): δ7.95(d, 1H), 7.62(d, 1H), 7.15(s, 1H), 6.34(s, 1H), 6.12(s, 1H), 5.31(s, 1H), 5.12(s, 1H), 4.54(s, H), 4.38(dt, 1H), 4.07(dd, H), 3.56(dt, H), 2.84(q, H), 2.61(s, H), 2.30(s, H ), 2.18(s,H), 2.13(s,H), 1.88(s,H), 1.73(s,H), 1.10(s,H), 1.04(s,H).
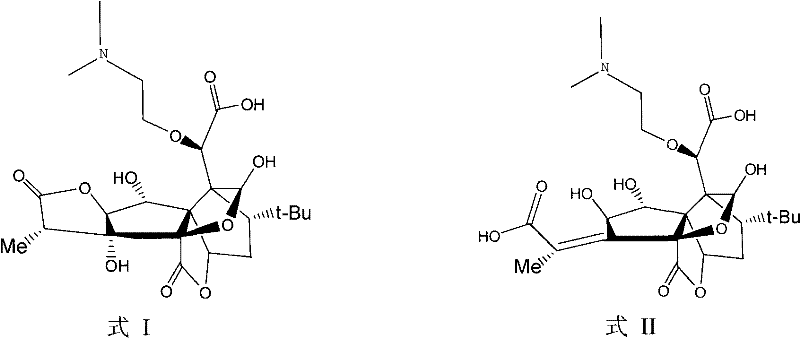
[0024] The compound D obtained in Example 2 is the same as the compound B obtained in Example 1, and its molecular structure is shown in Formula 2. MS(m / z): 514[M+H] +1 ; 1 H-NMR (CDCl 3 ): δ7.88(d, 1H), 7.60(d, 1H), 7.42(d, 1H), 7.12(s, 1H), 6.74(s, 1H), 6.04(s, 1H), 5.27(s, 1H), 5.04(s, 1H), 4.51(s, H), 4.29(dt, 1H), 4.11(dd, H), 3.48(dt, H), 2.71(s, H), 2.26(s, H ), 2.12(s,H), 2.01(s,H), 1.87(s,H), 1.76(s,H), 1.13(s,H), 1.02(s,H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com