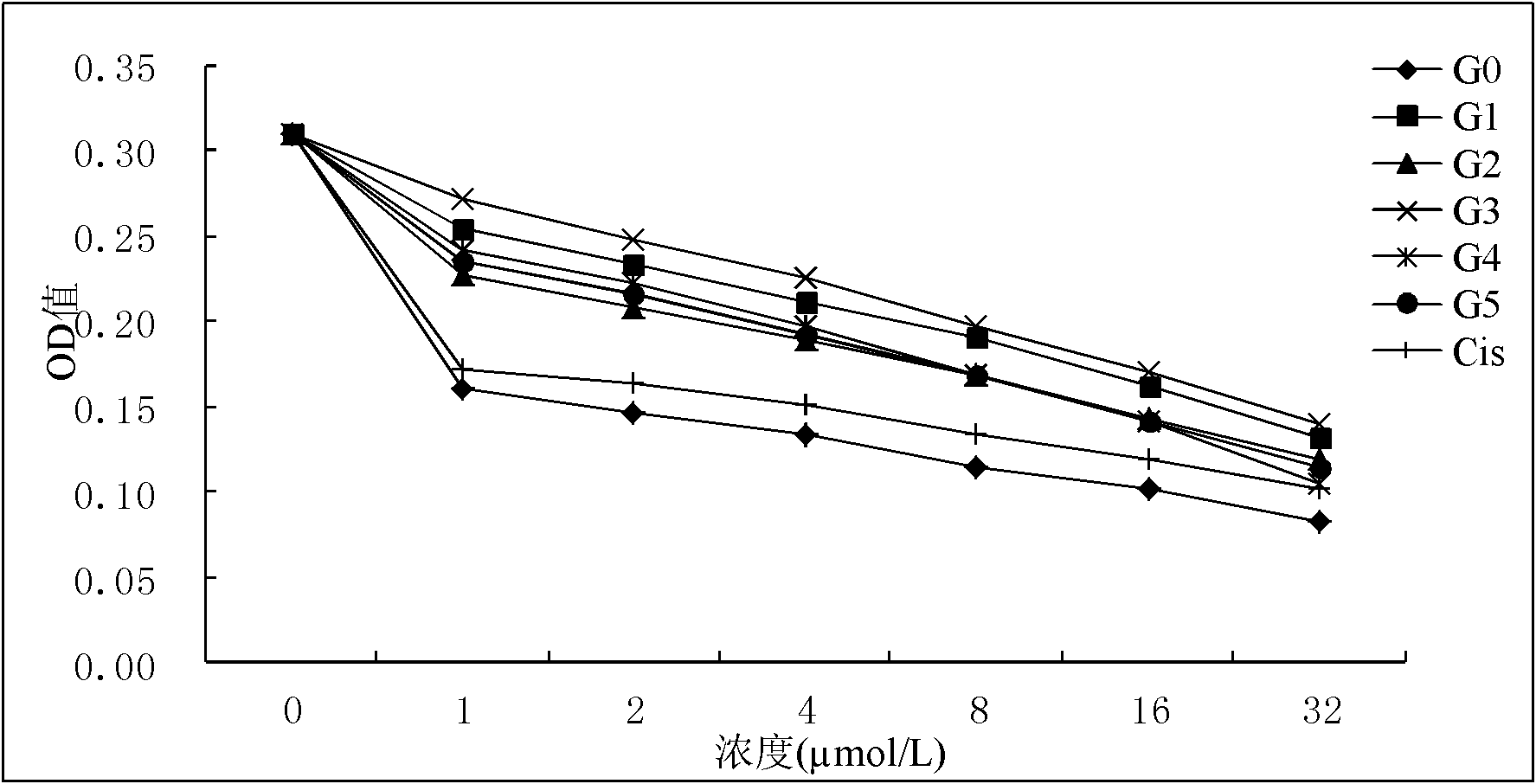
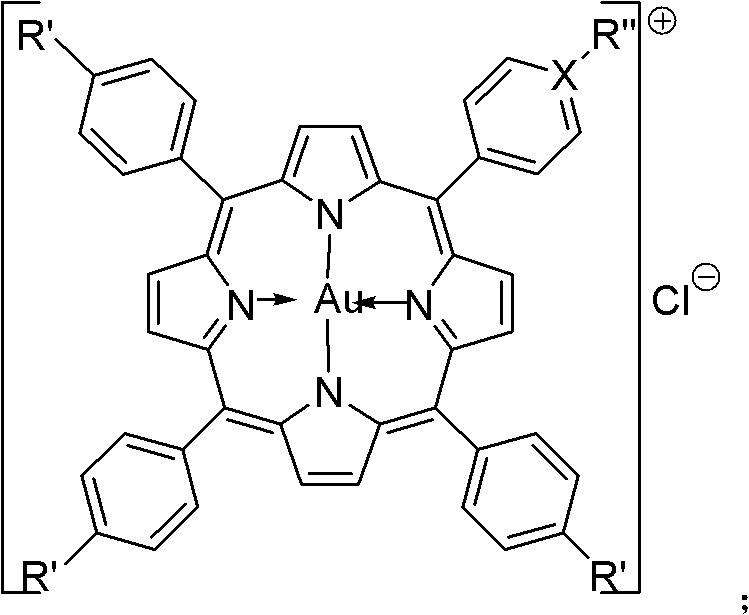
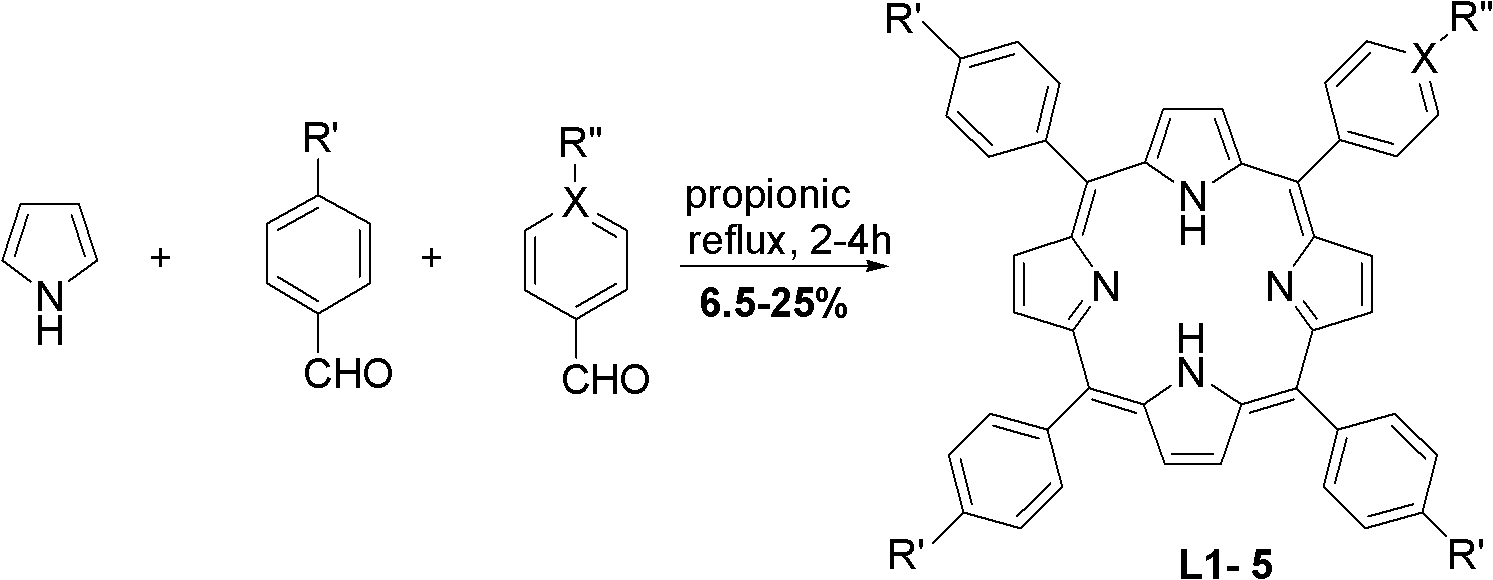
Substituted porphyrin gold (III) compounds with anticancer activity and preparation method thereof
An anti-cancer activity and compound technology, applied in the fields of organic chemistry, drug combination, anti-tumor drugs, etc., can solve the problems of large distance, poor targeting selectivity, high toxicity, etc., achieve good anti-cancer activity, small toxic side effects, scientific reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
[0027] Example 15-(4-p-methoxyphenyl)-10,15,20-triphenylporphyrin gold (G1) compound and its preparation method.
[0028] Add 200mL of propionic acid, benzaldehyde (60mmol) and p-methoxybenzaldehyde (20mmol) into a 250mL round bottom flask, and heat to reflux. After mixing 5.53mL (80mmol) freshly distilled pyrrole and 10mL propionic acid, slowly add it dropwise to the propionic acid solution within 30min. Then the reaction was continued for 1 h, cooled to room temperature, a solid was precipitated, filtered, the solid was washed with methanol and hot water, and dried. 5-(4-p-methoxyphenyl)-10,15,20-triphenylporphyrin (L1) was obtained in a yield of 8.8%.
[0029] Add 5-(4-p-methoxyphenyl)-10,15,20-triphenylporphyrin (L1) (0.025mmol), potassium chloroaurate (0.07-0.08mmol), sodium acetate ( 0.25-0.35mmol) and 7.5-15.25mL glacial acetic acid, conventionally heated to reflux for 3-4h. TLC monitored until the reaction was complete. Acetic acid was distilled off in a rotary eva...
Embodiment 2
[0030] Example 2 Chlorination of 5,10,15,20-tetrakis(4-methoxycarbonyl)phenylporphyrin gold (G2) compound and its preparation method.
[0031] Add 200 mL of propionic acid and 4-methoxycarbonylbenzaldehyde (80 mmol) into a 250 mL round bottom flask, and heat to reflux. After mixing 5.53mL (80mmol) freshly distilled pyrrole and 10mL propionic acid, slowly add it dropwise to the propionic acid solution within 30min. Then the reaction was continued for 1 h, cooled to room temperature, a solid was precipitated, filtered, the solid was washed with methanol and hot water, and dried. 5,10,15,20-tetrakis(4-methoxycarbonyl)phenylporphyrin (L2) was obtained with a yield of 22.0%: mp>250°C; 1 H NMR (600MHz, CDCl 3 ), δ (ppm): -2.79 (s, 2H, inner-NH), 4.11 (s, 12H, -COOCH 3 ), 8.29 (d, J=7.8Hz, 8H, Ph-CH), 8.44 (d, J=7.8Hz, 8H, Ph-CH), 8.81 (s, 8H, Por-CH); IR(KBr): υ3425(m), 2919(w), 1724(s), 1607(w), 1435(w), 1383(w), 1277(m), 1108(m), 965(w), 803(w), 762(w)cm -1 ; UV-Vis (CH 2 Cl ...
Embodiment 3
[0033] Example 3 Chlorinated 5-(4-methoxycarbonyl)phenyl-10,15,20-triphenylporphyrin gold (G3) compound and its preparation method.
[0034] Using the method of Example 1, replace p-methoxybenzaldehyde (20mmol) with 4-methoxycarbonylbenzaldehyde (20mmol) to participate in the reaction, and obtain 5-(4-methoxycarbonyl)phenyl- 10,15,20-Triphenylporphyrin compound (L3). mp>250°C; 1 H NMR (CDCl 3 , 600MHz), δ (ppm): -2.79 (s, 2H, inner-NH), 4.11 (s, 3H, -COOCH 3 ), 7.74-7.79 (m, 9H, Ph-CH), 8.21 (d, J=6.6Hz, 6H, Ph-CH), 8.31 (d, J=7.8Hz, 2H, Ph-CH), 8.44 (d , J=7.8Hz, 2H, Ph-CH), 8.79(d, J=4.2Hz, 2H, Por-CH), 8.85(s, 6H, Por-CH); IR(KBr): υ3442(s), 3319(w), 2924(w), 2853(w), 1812(m), 1722(s), 1604(w), 1472(w), 1437(w), 1394(w), 1353(w), 1279(s), 1182(w), 1106(w), 800(m), 739(w)cm -1 ; UV-Vis (CH 2 Cl 2 ) / nm 416, 513, 548, 589, 646.
[0035] Using the method of Example 1, replace 5-(4-p-methoxyphenyl)-10 with 5-(4-methoxycarbonyl)phenyl-10,15,20-triphenylporphyrin compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com