Synthesis method of 2'-deoxyguanosine by adopting nucleoside phosphorylase of brevibacterium acetylium
A technology of nucleoside phosphorylase and Brevibacterium acetyl, applied in the field of synthesizing 2'-deoxyguanosine, can solve the problems of long route and high cost, and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Put 50ml of medium in a 250ml Erlenmeyer flask, which contains 1% beef extract, 1% peptone, 0.5% yeast extract, 0.5% NaCl, pH 7.0, sterilize at 118°C for 30min, and insert Brevibacterium acetyl QD96 after cooling , shake (200r / min) at 30°C for 16h, freeze and centrifuge the culture solution at 4000r / min for 30min, wash with 0.05mol / L phosphate buffer (pH7.0), and centrifuge again, and store the obtained bacteria in cold storage , as a nucleotide phosphorylase.
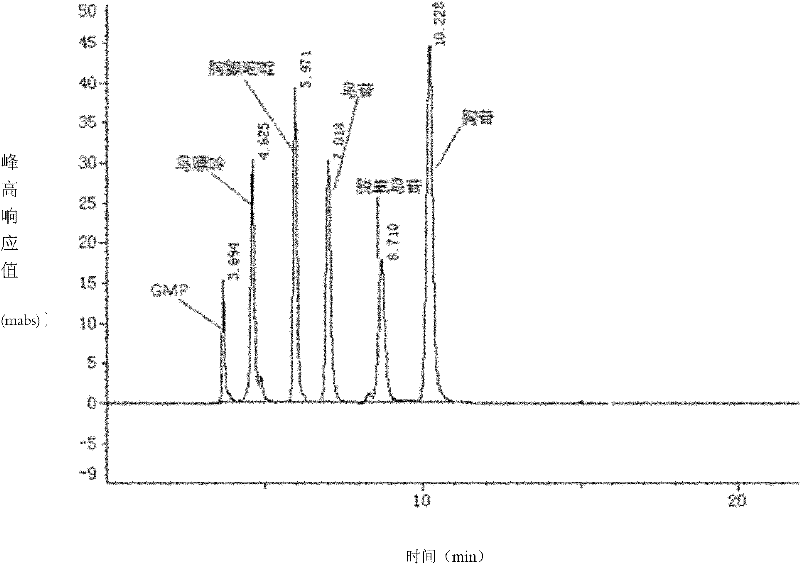
[0025] In the 100ml round bottom flask, pack 50ml substrate solution, wherein contain guanylic acid and thymidine concentration and be respectively 40mol / L, phosphate buffer is 25mmol / L, thalline addition is 5% (wet weight), in Stir and react in a constant temperature water bath at 60° C. for 2 hours. After the reaction is completed, the cells and a small amount of thymine precipitates are removed by refrigerated centrifugation. Then the solution is detected by HPLC to contain 24mmol / L deoxyguanosine, and its c...
Embodiment 2
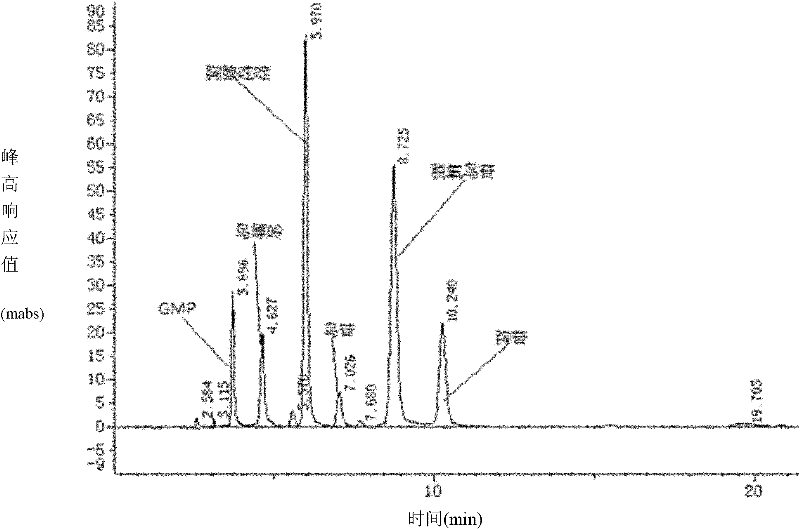
[0028] In the 100ml round-bottomed flask, pack 50ml substrate solution, wherein contain guanylic acid and 2'-deoxyuridine concentration and be respectively 40mmol / L, phosphate buffer is 25mmol / L, and thalline addition is 5% (wet weight ), and then operate with embodiment 1. After the reaction, the solution was detected by HPLC, which contained 26 mmol / L deoxyguanosine, and its conversion rate was 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com