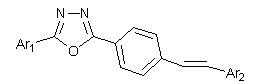
Stilbene derivative with 1,3,4-oxadiazole and preparation method and application thereof
A technology of stilbene and its derivatives, which is applied in the field of pesticide chemistry and fine chemicals, can solve the problems of narrow application range, harsh synthesis conditions, and inconspicuous effects of a single active structural unit, and achieve significant insect growth and development inhibitory activity, synthetic Easy operation and environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
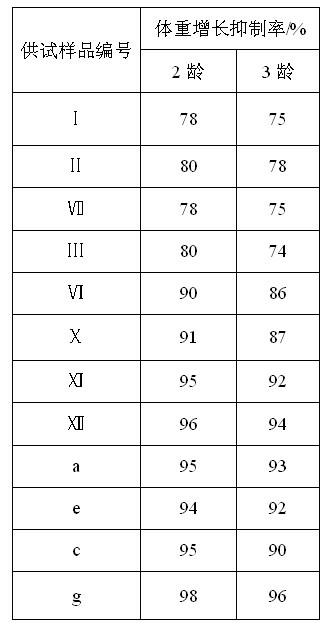
Examples
Embodiment 1
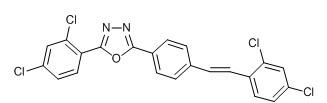
[0034] Example 1 Synthesis of 2-phenyl-5-(4-(3,4-dichlorostyryl)phenyl)-1,3,4-oxadiazole (Ⅰ)
[0035]
[0036] (I)
[0037] (1) Dissolve 30.0g (0.20mol) of p-toluylhydrazide in 700ml of absolute ethanol, add 21.2g (0.20mol) of benzaldehyde, heat and stir under reflux for 0.5h to obtain a reaction solution, cool and filter the reaction solution, and dry the filter cake to obtain 42.0g white solid; weigh 23.8g (0.10mol) of the white solid and dissolve it in 500ml absolute ethanol, then add 85g (0.30mol) chloramine T, heat and reflux and stir for 3h to obtain the intermediate reaction solution , the remaining solid after ethanol was evaporated was washed with distilled water and filtered 4 times, and the filter cake was dried, and the resulting dry filter cake was recrystallized with a mixed solution of distilled water and acetone with a volume ratio of 1:1 to obtain 15.75g 2-phenyl-5- (4-Benzyl)-1,3,4-oxadiazole, its yield: 66.7%. Melting point: 129°C. Proton NMR structu...
Embodiment 2
[0041] Example 2 Synthesis of 2-phenyl-5-(4-(2,4-dichlorostyryl)phenyl)-1,3,4-oxadiazole (Ⅱ)
[0042]
[0043] (II)
[0044] Reaction steps (1), (2), (3) are the same as in Example 1, and the recrystallization product of step (3) gained is the same as that of Example 1;
[0045] (4) 1.0 g (2.69 mmol) of 4-(5-phenyl-1,3,4-oxadiazol-2-yl) benzyl phosphonate prepared in step (3) and 0.47 g ( 2.69mmol) 2,4-dichlorobenzaldehyde was dissolved in 10ml N,N-dimethylformamide to obtain a solution, then in the solution, dropwise added anhydrous potassium tert-butoxide with a mass percentage of 20%. 6ml of ethanol solution, stirred and reacted at 60°C for 5 h to obtain a reaction solution, cooled and filtered the reaction solution, and the resulting filter cake was recrystallized with a mixture of dimethyl sulfoxide and ethanol at a volume ratio of 7:1 to obtain the final product. Yield: 91.2%. Melting point: 213.8°C. Proton NMR structural characterization data: 1H NMR (400 MHz, ...
Embodiment 3
[0047] Example 3 Synthesis of 2-(2,4-dichlorophenyl)-5-(4-styrylphenyl)-1,3,4-oxadiazole (Ⅲ)
[0048]
[0049] (Ⅲ)
[0050] (1) Dissolve 15.0g (0.10mol) of p-toluylhydrazide in 300ml of absolute ethanol, add 17.5g (0.10mol) of 2,4-dichlorobenzaldehyde, heat and stir under reflux for 1 hour to obtain a reaction solution, and cool And filter the reaction solution, dry the filter cake to obtain 29.5g white solid; weigh 29.0g (0.095mol) white solid and dissolve it in 500ml absolute ethanol, then add 136g (0.48mol) chloramine T, heat, stir and reflux for 5h The intermediate reaction solution was obtained, and the remaining solid was washed with distilled water and filtered 5 times after the ethanol was evaporated, and the obtained filter cake was dried, and the dried product was recrystallized with a mixed solution of distilled water and acetone with a volume ratio of 1:2 to obtain 15.59 g of 2- (2,4-Dichlorophenyl)-5-(4-benzyl)-1,3,4-oxadiazole. Yield: 53.9%. Melting poi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com