Bispyridyl benzimidazole compounds and synthesis method thereof
A technology of bispyridylbenzimidazole and pyridylbenzimidazole, which is applied in the field of bispyridylbenzimidazole compounds and their synthesis, can solve problems such as the lack of blue light materials, hindering cathode electron injection, and restricting the development of organic full-color displays. , to achieve the effect of high product yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
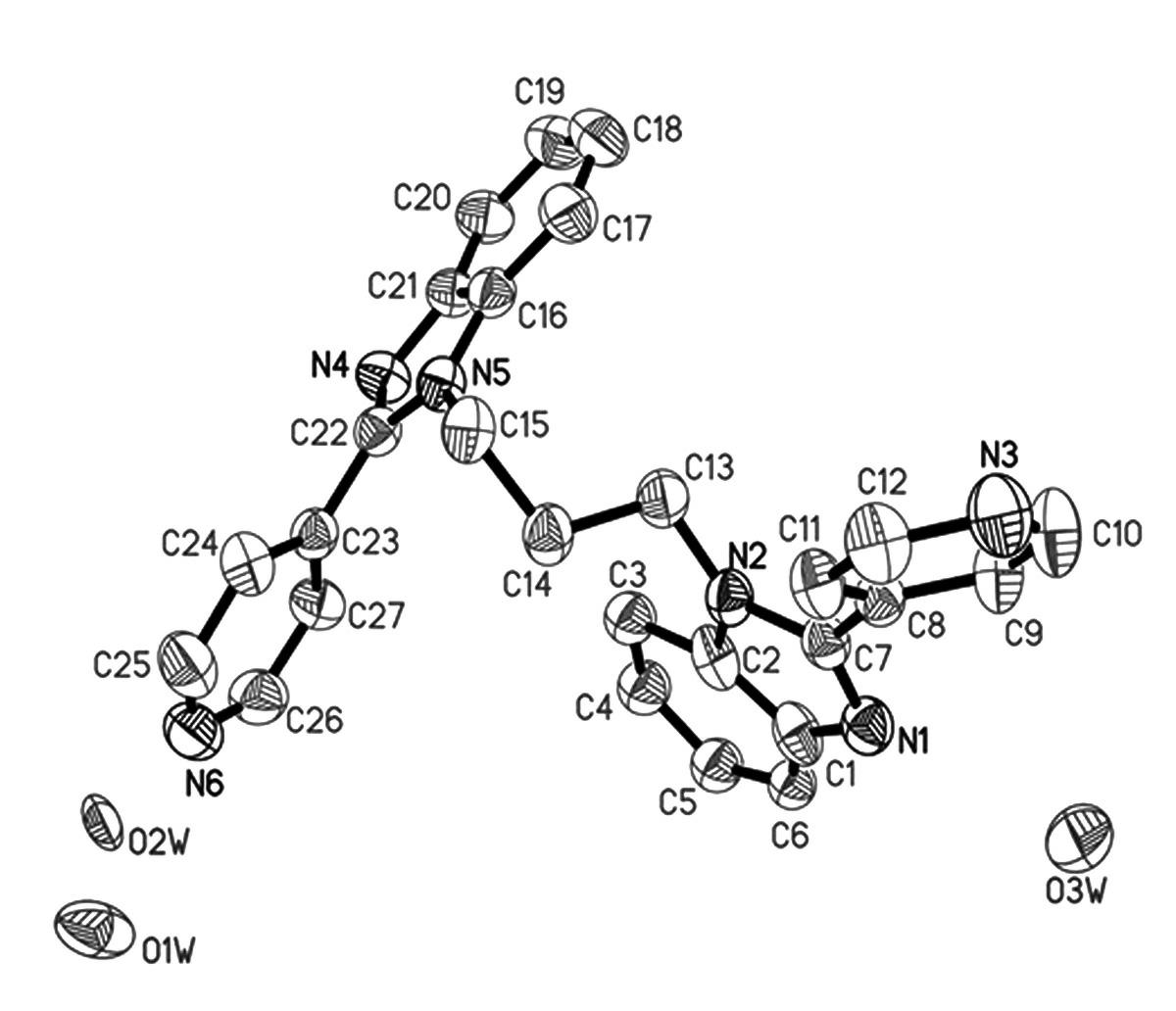
Examples
Embodiment 1
[0019] Embodiment 1: the preparation of 2-(2-pyridine)-benzimidazole
[0020] Add 4.92g (40mmol) of 2-pyridinecarboxylic acid, 4.32g (40mmol) of o-phenylenediamine and about 15mL of polyphosphoric acid into a 50mL beaker. 4 times, about 1.5 min each time, and then pour the obtained dark green solution into ice water, and a blue precipitate is formed. After filtering, the obtained precipitate was dispersed in water, and the pH value was adjusted to about 9 to obtain a white precipitate. Dissolve the precipitate in ethanol solution, add activated carbon for decolorization, and then recrystallize in a mixed solvent of V (ethanol): V (water) = 1:3 to obtain 2-pyridinebenzimidazole with a yield of 88%.
[0021] The preparation method of 2-(3-pyridine)-benzimidazole and 2-(4-pyridine)-benzimidazole compound is exactly the same as that of Example 1, and the yields are 85% and 80% respectively.
Embodiment 2
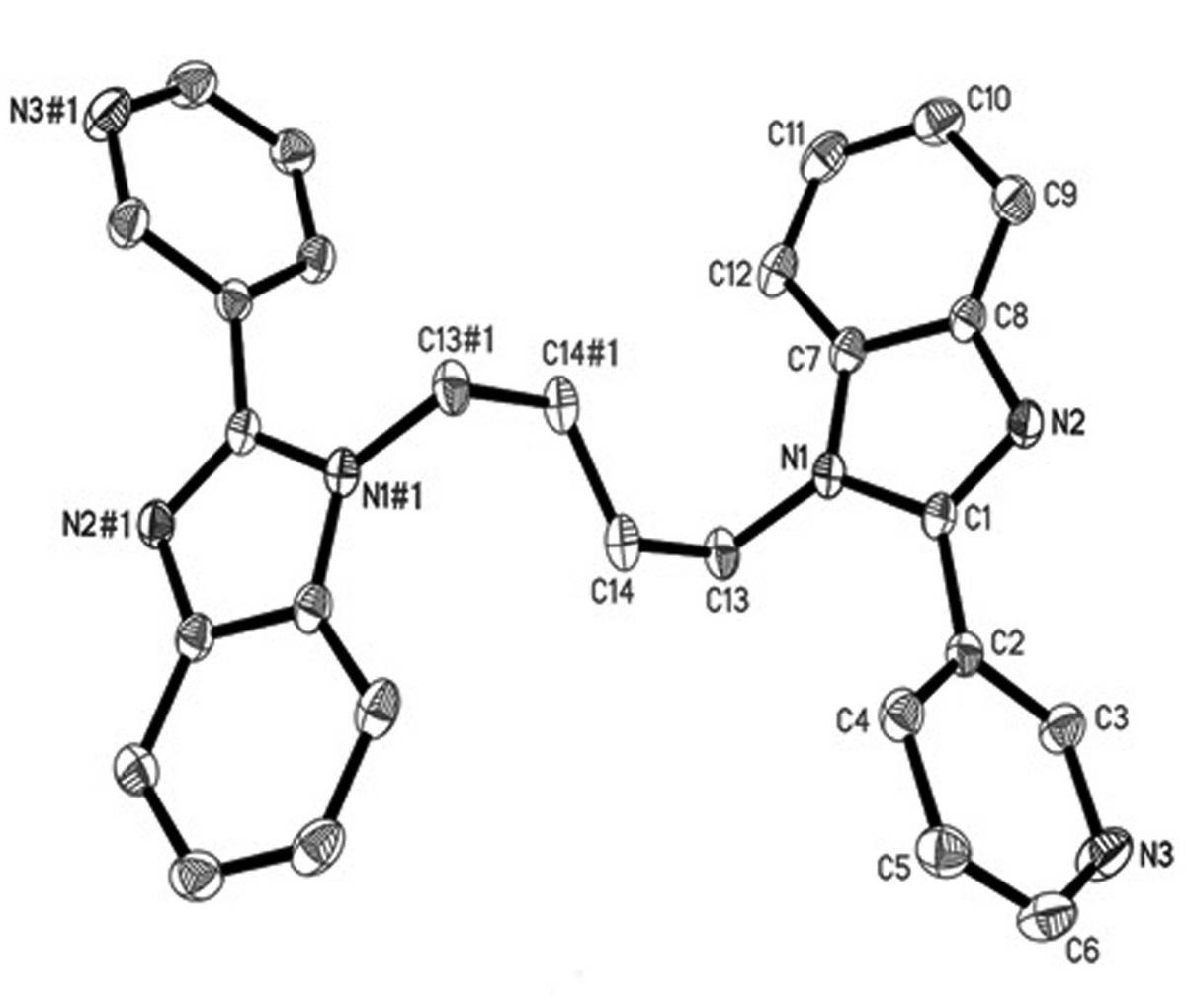
[0022] Example 2: Preparation of 1,1'-(1,4-butyl)bis[2-(3-pyridine)benzimidazole] (Compound 1 for short)
[0023] In a dry and clean 100mL flask, add 7.8g (40 mmol) of 2-(3 pyridyl) benzimidazole and 2.4g (60 mmol) of sodium hydroxide, then add 30 mL of DMF, and raise the temperature to 60 o C, after stirring for about 30 min, add 4.32 g (20 mmol) of 1,4-dibromobutane, continue to raise the temperature to 80 ° C, react for 8 h, remove most of the DMF solvent from the product under reduced pressure, pour into 400 mL of ice water, a light yellow solid was formed, and the resulting precipitate was recrystallized in a mixed solvent of V (methanol): V (water) = 1:3 after filtration to obtain a light yellow crystal of compound 1 with a yield of 75 %. Elemental Analysis C 28 h 24 N 6 (Mr = 444.53) C, 75.65; H, 5.44; N, 18.91. Actual value: C, 75.72; H, 5.39; N, 18.84. IR (cm -1 ): 3389(ms), 3061(ms), 2956(s), 1948(ws), 1899(ws), 1603(s), 1569(s), 1509(ms), 1451(s), 1421(s ),...
Embodiment 3
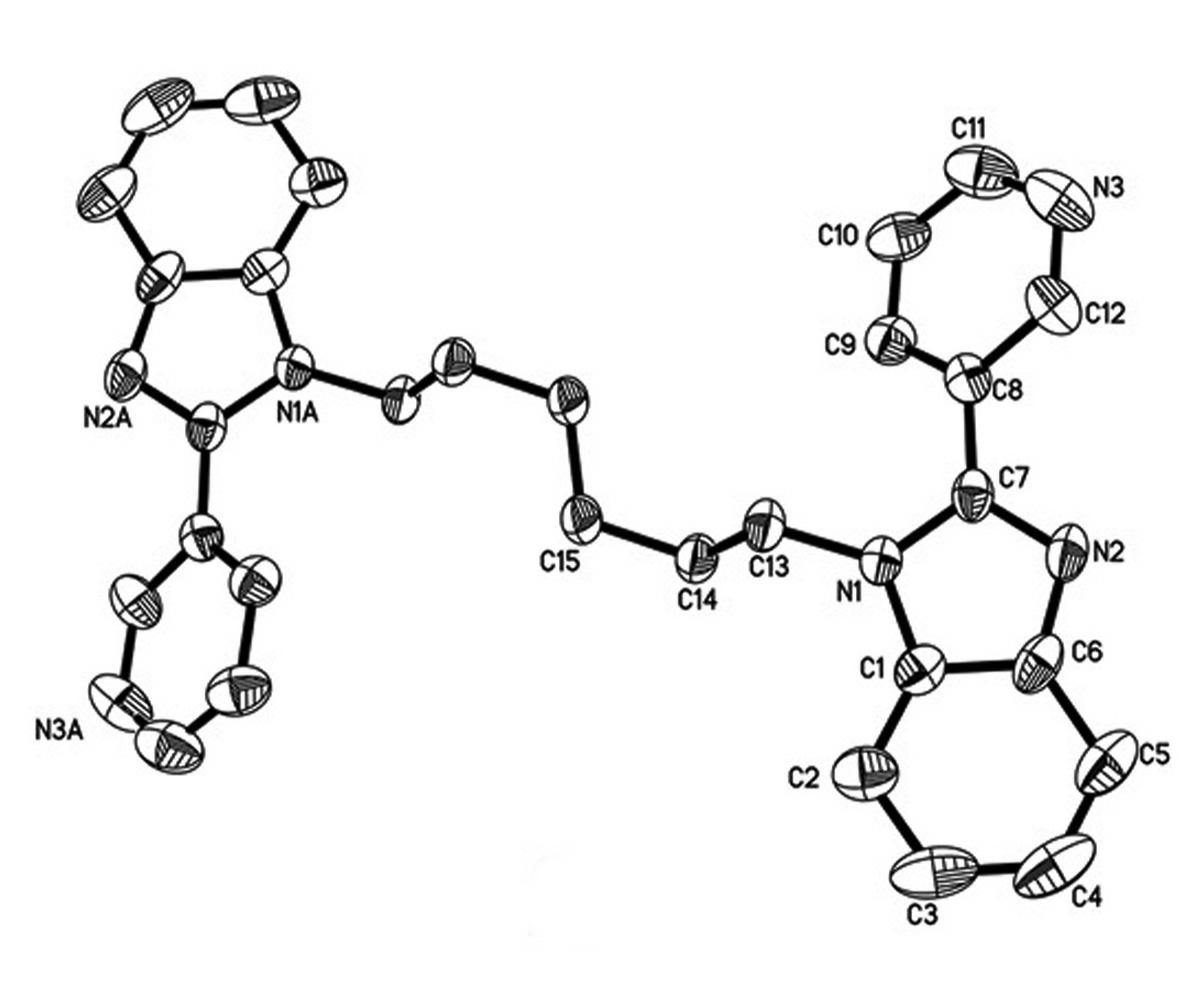
[0024] Example 3: Preparation of 1,1'-(1,6-hexyl)bis[2-(3-pyridine)benzimidazole] (Compound 2 for short)
[0025] Compound 2 was synthesized according to the preparation method of compound 1, except that 1,4-dibromobutane was replaced by 1,6-dibromohexane, and the reaction time was increased from 8h to 10h to obtain brown compound 2 with a yield of 72 %. Elemental Analysis C 30 h 28 N 6 (Mr =472.568) C, 76.24; H, 5.97; N, 17.79. Actual value: C, 75.99; H, 5.89;N, 17.86. IR (cm -1 ): 3378(ms), 3058(ms), 2936(s), 1886(ms), 1596(s), 1565(ms), 1507(ms), 1463(s), 1431(s), 1382(s ), 1363(s), 1332(s), 1275(s), 1252(s), 1194(s), 1158(s), 1108(s), 1082(s), 1011(s), 986(s) ), 924(s), 829(s), 757(ms), 701(s), 626(s), 579(s), 428(ms).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com