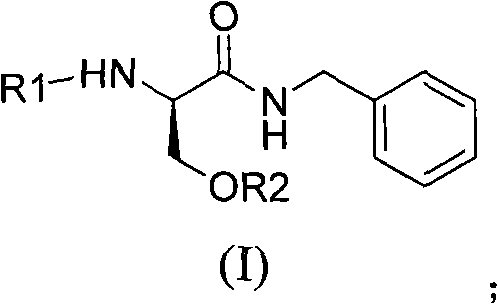
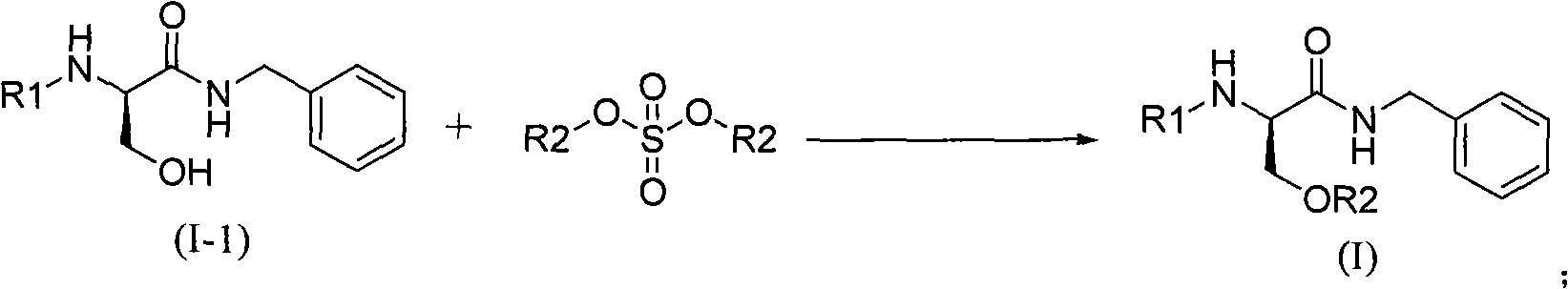
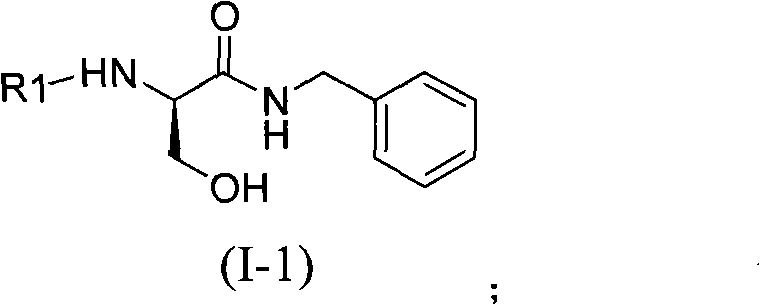Lacosamide intermediate compound and preparation method and application thereof
A compound, a phthalimide-based technology, is applied to the intermediate compound of the drug lacosamide and its preparation field, which can solve the problems of high price, unfavorable industrial production, and high production cost, and achieve low production cost and easy Industrialized production, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 (R)-N-benzyl-2-(tert-butoxycarbonyl amino)-3-hydroxyl propionamide
[0050]
[0051] Dissolve 32.16g (0.30mol) of benzylamine in 150g of anhydrous ethyl acetate to prepare a solution for later use.
[0052] Add 51.5g (0.25mol) (R)-2-(tert-butoxyamino)-3-hydroxypropionic acid and 450g anhydrous ethyl acetate in sequence to a 1000ml four-neck round bottom flask, and start mechanical stirring. Cool down to -10°C. 27.83 g (0.275 mol) of N-methylmorpholine and 37.59 g (0.275 mol) of isobutyl chloroformate were added to the system. After the addition, add the above-prepared benzylamine-ethyl acetate solution (32.16 g of benzylamine dissolved in 150 g of anhydrous ethyl acetate) at -15 to -10°C. Subsequently, the temperature was raised to 10-15° C., and the reaction was carried out with heat preservation. After the reaction is complete, add 200 g of tap water and stir for several minutes. Stand to separate layers and take the organic phas...
Embodiment 2
[0053] The preparation of embodiment 2 (R)-2-amino-N-benzyl-3-hydroxyl propionamide
[0054]
[0055] Add 34.0g (R)-N-benzyl-2-(tert-butoxycarbonylamino)-3-hydroxypropionamide and 350ml dichloromethane into a 500ml four-neck flask, stir to dissolve. Add 40.0 g of concentrated hydrochloric acid (mass concentration: 36.0%) at 20-25°C. Continue to react when done. TLC tracking, until the conversion of raw materials is complete. After the reaction was completed, the temperature was lowered to 20°C. After suction filtration, white needle crystals were obtained. The resulting white crystalline solid was dissolved in 50 g of water. Add 30% sodium hydroxide solution at 20-25°C to adjust the pH of the system to 8-9. After the addition, the stirring was continued, and white flaky crystals were precipitated. After suction filtration, washing and drying, 16.5 g (0.085 mol) of the target product was obtained with a molar yield of 73.52%.
Embodiment 3
[0056] The preparation of embodiment 3 (R)-N-benzyl-2-(ethoxycarbonylamino)-3-hydroxyl propionamide
[0057]
[0058] Add 9.65g (0.0497mol) (R)-2-amino-N-benzyl-3-hydroxypropionamide, 120ml tetrahydrofuran and 7.58g (0.07455mol) triethylamine to a 250ml three-necked flask, and stir to dissolve. Add 6.01 g (0.0554 mol) ethyl chloroformate at room temperature. After the reaction was completed, the solvent was evaporated to dryness, 100ml of water was added to the residue, and the mixture was stirred evenly. Extract with 50ml×3 ethyl acetate, combine the organic phases, and dry over anhydrous sodium sulfate. The solvent was evaporated to dryness to obtain 9.67 g (0.0363 mol) of white solid with a molar yield of 73.04%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com