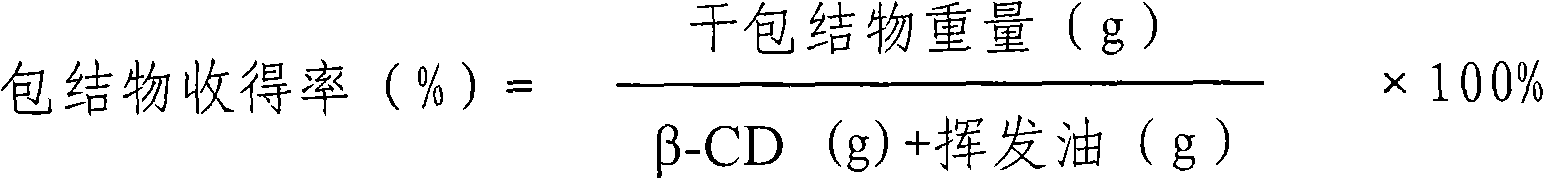Serial Chinese medicinal preparation for treating child common cold and preparation process and quality control method thereof
A technology of traditional Chinese medicine preparations and children's colds, which is applied in the direction of medical formulas, drug combinations, respiratory diseases, etc., can solve the problems of loss of volatile oil and active ingredients, affect clinical curative effect, and affect curative effect, so as to reduce process and turnover and ensure clinical curative effect , the effect of shortening the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
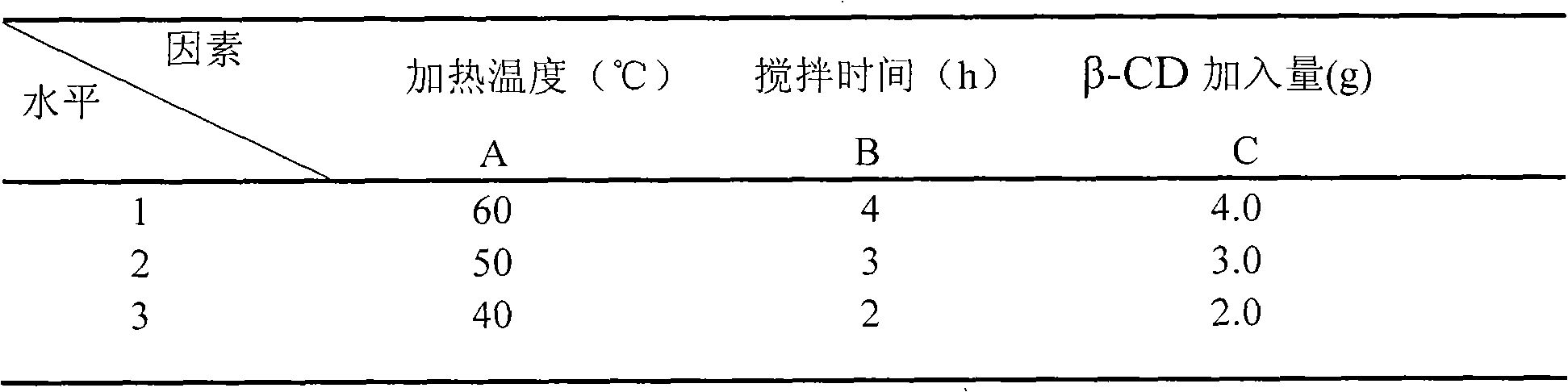
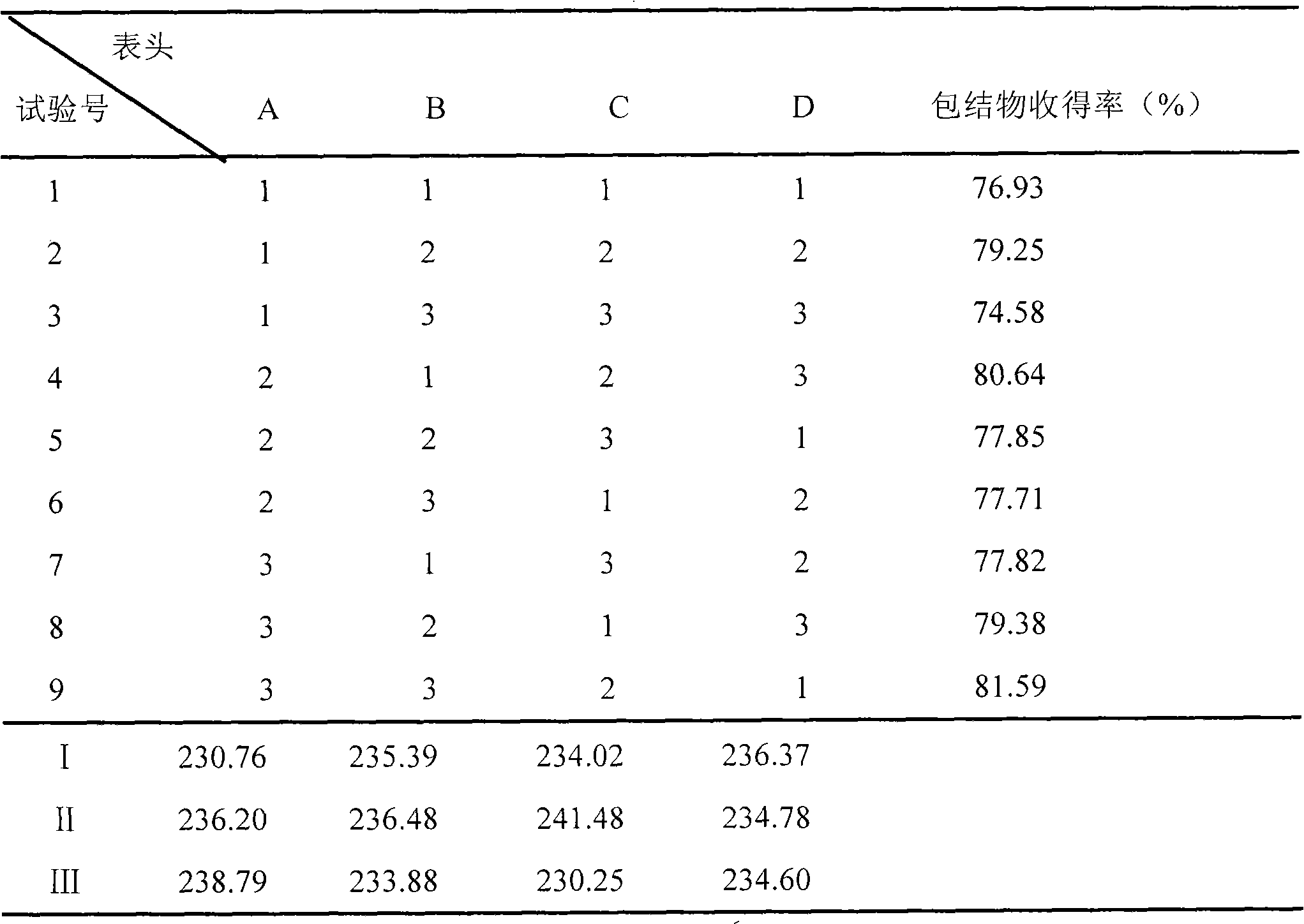
[0113] Take 125 parts by weight of Campanulaceae and pulverize into fine powder; add 415 parts by weight of Bupleuri, add 6 times the amount of water, soak for 6 hours, distill and extract the volatile oil for 3 hours, collect the distilled volatile oil distillate and aqueous solution in another device; take the distillate of volatile oil, add β -Cyclodextrin (100ml:1g), stirred at 30°C for 4h, clathrate into clathrate; 830 parts by weight of medicinal residues and Folium baicalensis, 415 parts by weight of Scutellaria baicalensis, 415 parts by weight of Schizonepeta, 125 parts by weight of bellflower, 165 parts by weight of licorice Mix the parts by weight, add water to decoct twice, add 6 times the amount of water to decoct twice (3, 2 hours in sequence), combine the decoction and the distilled aqueous solution of Bupleurum radix, filter, and concentrate the filtrate until it is measured at 60-80°C. To obtain a concentrated solution with a relative density of 1.20-1.25, add e...
Embodiment 2
[0115] Take 125 parts by weight of Campanulaceae and pulverize into fine powder; add 415 parts by weight of Radix Bupleurum, add 6 times the amount of water, soak for 6 hours, distill and extract the volatile oil for 5 hours, collect the distilled volatile oil distillate and aqueous solution in another device; take the distillate of volatile oil, add β -Cyclodextrin (100ml: 6g), stirred at 60°C for 2h to form an inclusion compound; 830 parts by weight of medicinal residues and Folium baicalensis, 415 parts by weight of Scutellaria baicalensis, 415 parts by weight of Schizonepeta, 125 parts by weight of platycodon grandiflorum, 165 parts by weight of licorice Mix the parts by weight, add water to decoct twice, add 6 times the amount of water to decoct twice (3, 2 hours in sequence), combine the decoction and the distilled aqueous solution of Bupleurum radix, filter, and concentrate the filtrate until it is measured at 60-80°C. To obtain a concentrated solution with a relative de...
Embodiment 3
[0117] Take 125 parts by weight of Campanulaceae and pulverize into fine powder; add 415 parts by weight of Bupleurum, add 6 times the amount of water, soak for 6 hours, distill and extract the volatile oil for 5 hours, collect the distilled volatile oil and aqueous solution in another device; take the volatile oil, add β-cyclodextrin In aqueous solution (4%) (2g: 100ml), stirred at 30°C for 4h to form clathrates; 830 parts by weight of medicinal residues and Folium Folium, 415 parts by weight of Scutellaria baicalensis, 415 parts by weight of Schizonepeta, 125 parts by weight of Campanulaceae, Mix 165 parts by weight of licorice, add water to decoct twice, add 8 times the amount of water to decoct twice (3 and 2 hours in turn), combine the decoction and the distilled aqueous solution of Bupleurum radix, filter, and concentrate the filtrate to 60-80 The concentrated solution with a relative density of 1.20-1.25 was measured at ℃, added ethanol to make the alcohol content 70%, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com