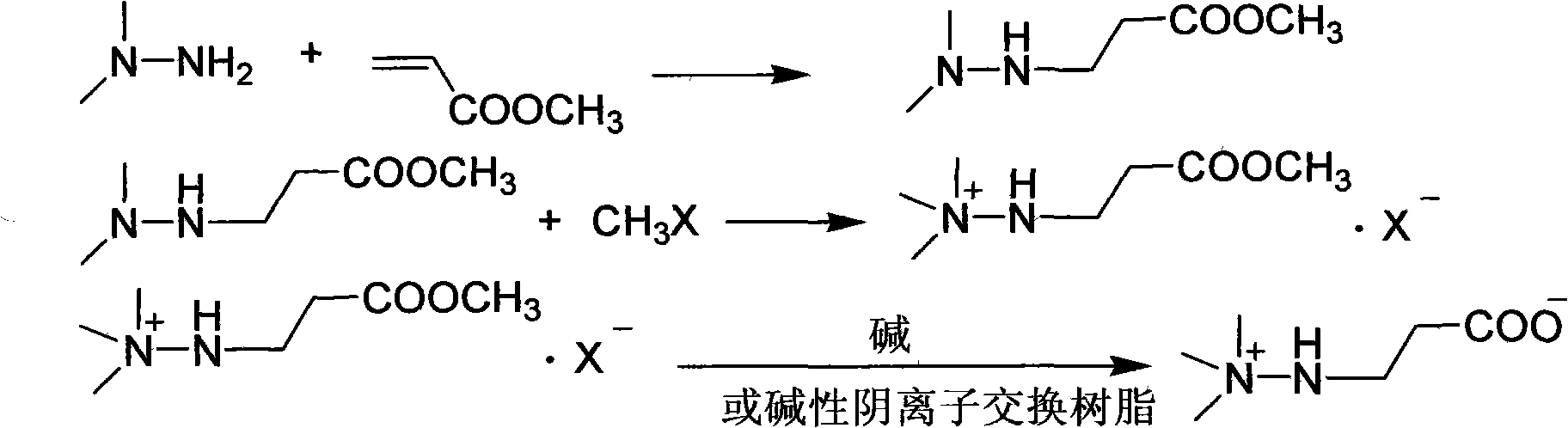
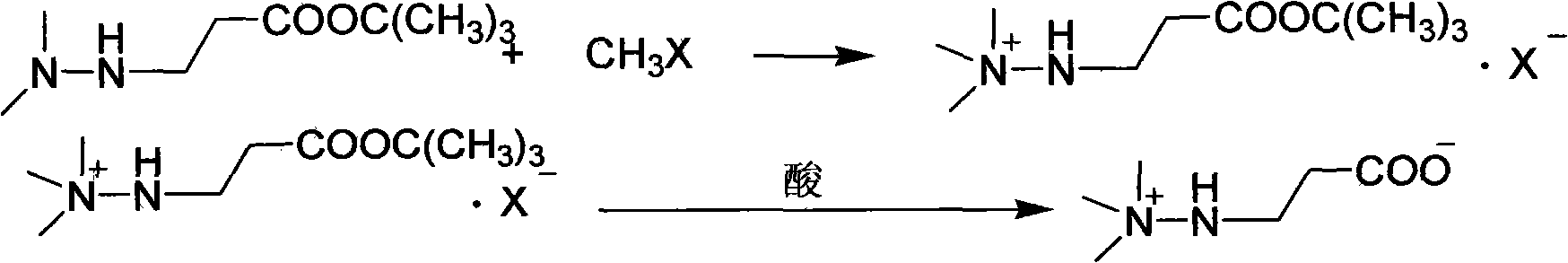
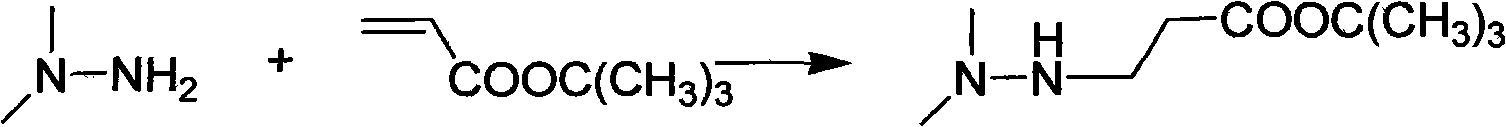Preparation method of 3-(2,2,2-trimethylhydrazine)propionate dihydrate
A technology of trimethylhydrazine and dihydration, applied in the field of preparation of dihydrate 3-propionate, can solve the problems of long filling process, difficult removal, low efficiency, etc., and achieve the effect of less dosage and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 128 g (1 mol) of tert-butyl acrylate into a three-necked flask with mechanical stirring and dropping funnel, cool to 0°C, add 63 g (1.05 mol) of unsymmetrical dimethylhydrazine dropwise, drop it in 1.5 hours, and continue at the same temperature After stirring for 1 hour, 171.1 g of tert-butyl 3-(2,2-dimethylhydrazine)propionate was distilled under reduced pressure, with a yield of 91% and a content of 98.8%;
[0030] Dissolve the above distillation product in 200ml of ethanol, cool to 0°C, pass through 90.8g of methyl chloride for 1 hour, continue stirring at the same temperature for 1 hour, filter, wash with cold ethanol, and dry to obtain 3-(2,2,2- Trimethylhydrazine) tert-butyl propionate chloride 234.3g, yield 91.0%, content 99.1%;
[0031] Dissolve the above-mentioned tert-butyl ester chloride in 100ml of water, add 10ml of 50% hydrochloric acid, heat and reflux for 2 hours, remove water and acid under reduced pressure, add 300ml of chloroform to the residue, ...
Embodiment 2
[0033] Add 128 g (1 mol) of tert-butyl acrylate into a three-necked flask with mechanical stirring and dropping funnel, cool to 0°C, add 66 g (1.1 mol) of unsymmetrical dimethylhydrazine dropwise for 1.5 hours, and continue at the same temperature After stirring for 1 hour, 172 g of tert-butyl 3-(2,2-dimethylhydrazine) propionate was distilled under reduced pressure, with a yield of 91.5% and a content of 98.7%;
[0034] Dissolve the above distillation product in 200ml of ethanol, cool to 0°C, pass through 96g of methyl bromide for 1 hour, continue stirring at the same temperature for 1 hour, filter, wash with cold ethanol, and dry to obtain 3-(2,2,2-trimethyl hydrazine) tert-butyl propionate bromide 236.3g, yield 91.3%, content 99.1%;
[0035] Dissolve the above-mentioned tert-butyl ester bromide in 100ml of water, add 10ml of 50% hydrobromic acid, heat to reflux for 3 hours, remove water and acid under reduced pressure, add 300ml of ethyl acetate to the residue, and adjust w...
Embodiment 3
[0037] The preparation of 3-(2,2-dimethylhydrazine) tert-butyl propionate is the same as in Example 1, except for step 2 and step 3;
[0038] Dissolve the above distillation product in 200ml of ethanol, cool to 10°C, pass through 135.7g of iodomethane for 1 hour, continue stirring at the same temperature for 1 hour, filter, wash with cold ethanol, and dry to obtain 3-(2,2,2- Trimethylhydrazine) tert-butyl propionate iodide 275.7g, yield 91.8%, content 99.0%;
[0039] Dissolve the above-mentioned tert-butyl ester iodide in 100ml of water, add 10ml of 50% hydroiodic acid, heat to reflux for 3 hours, remove water and acid under reduced pressure, add 300ml of dichloromethane to the residue, adjust the pH to 7-8 with trimethylamine, Stir for 10 minutes, filter, wash twice with dichloromethane, and recrystallize the solid with 95% ethanol to obtain 108.2 g of the target product, with a yield of 88.7% and a content of 99.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com