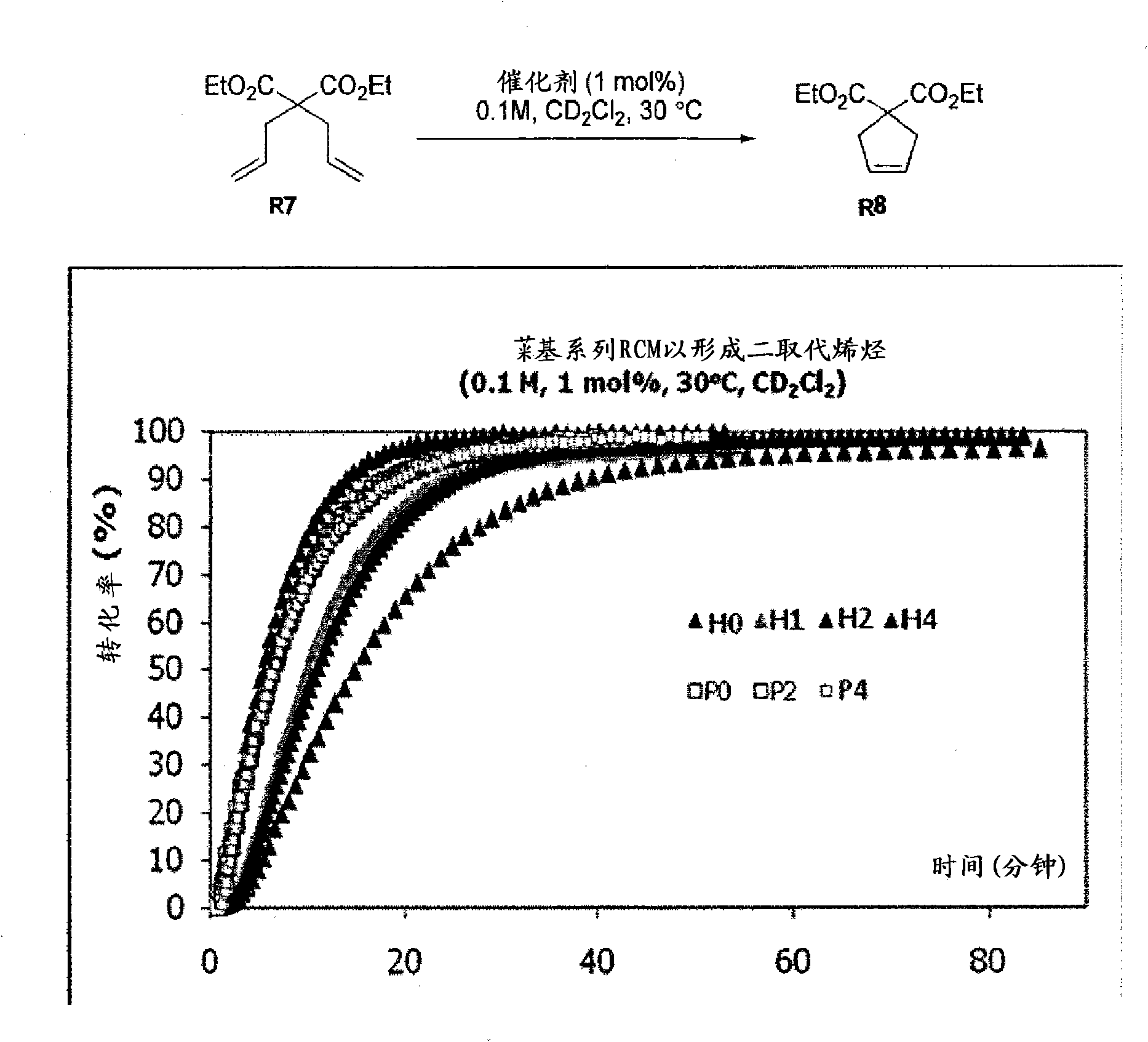
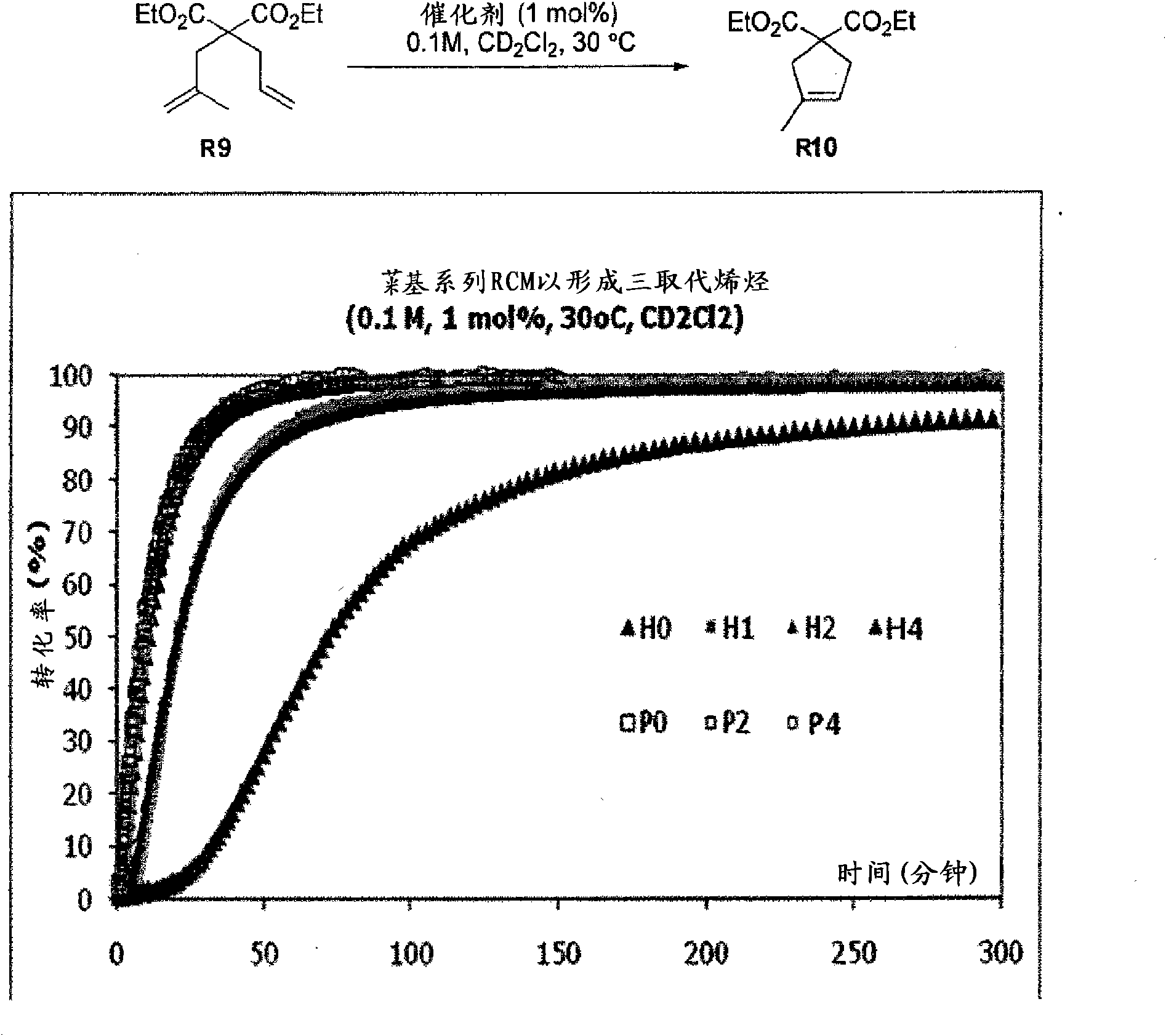
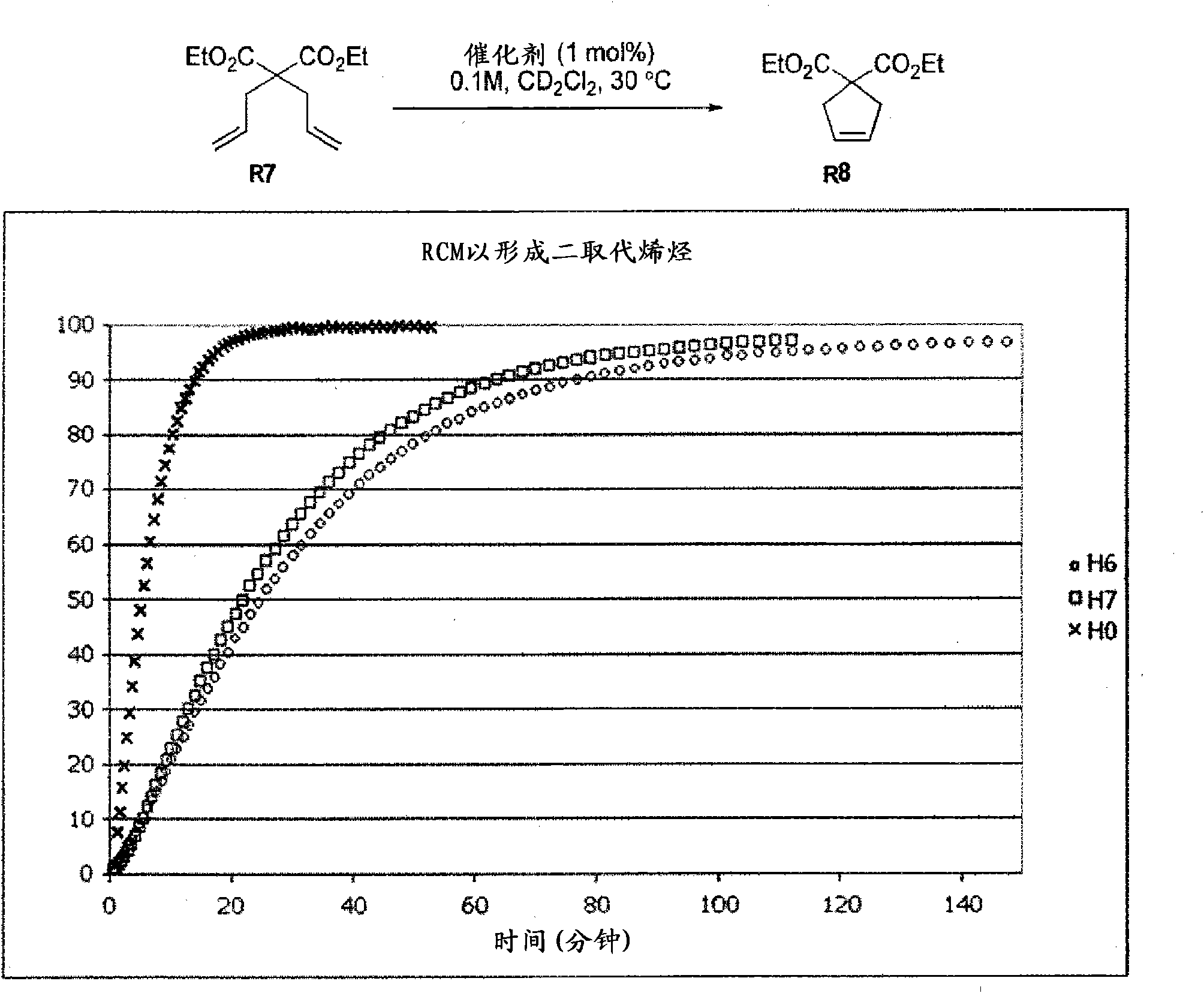
Ruthenium olefin metathesis catalysts bearing N-heterocyclic carbene ligands with substituted backbone
A carbocyclic and allyl-based technology, applied in organic chemistry, ruthenium organic compounds, platinum group organic compounds, etc., can solve problems such as limited service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] 2. Imidazoline salt preparation
[0068] Four-substituted or three-substituted imidazolines of formula (I) for forming the ruthenium catalyst of the present invention Salt NHC ligand precursors can be prepared from diamine derivatives bearing desired substituents and substitution patterns, as shown in the following examples. Typically, the diamine is dissolved in diethyl ether and treated with hydrogen chloride solution to precipitate the diamine hydrochloride. Diamine hydrochloride reacts with a large excess of triethyl orthoformate to give the desired imidazoline chloride of formula (I) Salt NHC ligand precursor. Alternatively, the diamine compound can also form a salt with trifluoroacetic acid or tetrafluoroboric acid, which likewise reacts with a large excess of triethylorthoformate to give the desired imidazoline of formula (I) Salt NHC ligand precursor.
[0069] 3. N-heterocyclic carbene (NHC) ruthenium catalyst of the present invention
[0070] The pres...
Embodiment 1
[0108] The preparation of embodiment 1.NHC ligand precursor (S):
[0109] Procedure A: A solution of the desired diamine in diethyl ether was treated with a solution of hydrogen chloride (2 equiv) to precipitate the diamine hydrochloride. The white solid was collected by filtration and washed with copious amounts of ether. The solid was placed in a flask and triethylorthoformate (large excess) was added. The resulting mixture was stirred at 130°C for 5 to 10 minutes and then cooled. After cooling to room temperature, the white solid was collected by filtration, washed with copious amounts of diethyl ether and then acetone to give the desired imidazoline chloride Salt S.
[0110] Procedure B: (see Jazzar, R.; Bourg, J.-B.; Dewhurst, R.D.; Donnadieu, B.; Bertrand, G.J.Org.Chem. 2007, 72, 3492-3499) The solution in hexane was added to a THF solution (40 mL) of the corresponding formamidine (1 equiv) at -78 °C. The mixture was stirred for 30 minutes, then allowed to warm to ...
Embodiment 1a
[0112] Example 1a.1,3-two yl-4-methyl-imidazoline chloride (S1) was prepared according to procedure B.
[0113]
[0114] 1 HNMR (500MHz, CDCl 3 ): δ9.70(s, 1H), 6.88(m, 4H), 5.02(m, 1H), 4.75(pseudo-t, J=11.5Hz, 1H), 3.85(dd, J=8.5Hz, J= 12.0Hz, 1H), 2.40-2.10(m, 18H), 1.50(d, J=6.5Hz, 3H). 13 CNMR (75MHz, CDCl 3 ): δ159.8, 140.4, 140.2, 135.8, 135.3, 135.1, 134.8, 130.4, 130.3, 130.2, 130.1, 130.0 (br s), 128.8, 60.5, 58.3, 21.1, 21.0, 19.0, 18.8, 18.5, 18.0 ( br s).
[0115] C 22 h 29 N 2 Calculated value of HRMS: 321.2331. Measured value: 321.2321.
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com