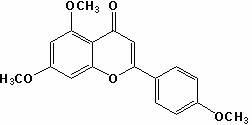
Method for preparing apigenin
A kind of apigenin and reprocessing technology, applied in the direction of organic chemistry, etc., to achieve the effect of abundant raw material sources, high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
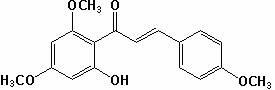
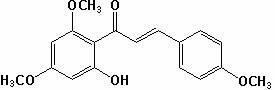
[0034] (1) Weigh 19.6g of 2,4-dimethoxy-6-hydroxyacetophenone, and the molar ratio of 2,4-dimethoxy-6-hydroxyacetophenone to p-methoxybenzaldehyde is 1 :1.2 Weigh 16.3g of p-methoxybenzaldehyde, add 356mL methanol to dissolve it according to the solid-liquid ratio of 1:10, then add sodium hydroxide to make the alkali concentration in the solution 15%, and stir at 20℃ 40h, then adjust the pH of the reaction solution to 3 with concentrated hydrochloric acid solution, filter the reaction solution with suction, wash the filter cake with distilled water, and dry the filter cake to obtain compound II; yield 90%; mp: 112~116; 1 HNMR (CDCl 3 ) Characterization: δ3.82(s, 3H), 3.85(s, 3H), 3.92(s,3H), 5.97(d,J=1.9Hz,1H), 6.11(d, J=1.9Hz, 1H), 6.93(d, J=7.5Hz, 2H), 7.56(d, J=7.5 Hz, 2H), 12.78(s, 1H);
[0035] (2) Take 15.7 g of compound II, add 15.7 mL of dimethyl sulfoxide with a solid-liquid ratio of 1:1, add 0.0785 g of iodine, heat to 150°C, stir for 3 hours, and cool; weigh 7.85 g of ...
Embodiment 2
[0038] (1) Weigh 19.6g of 2,4-dimethoxy-6-hydroxyacetophenone, and the molar ratio of 2,4-dimethoxy-6-hydroxyacetophenone to p-methoxybenzaldehyde is 1 :1. Weigh 13.6g of p-methoxybenzaldehyde, and add 332mL of ethanol according to the solid-liquid ratio of 1:10 to dissolve, then add potassium hydroxide to make the concentration of the alkali in the solution 10%, and stir at 40°C 20h, then adjust the pH of the reaction solution to 4 with concentrated hydrochloric acid solution, filter the reaction solution with suction, wash the filter cake with distilled water, and dry the filter cake to obtain compound II; yield 90%; mp: 112~116; 1 HNMR (CDCl 3 ) Characterization: δ3.82(s, 3H), 3.85(s, 3H), 3.92(s,3H), 5.97(d,J=1.9Hz,1H), 6.11(d, J=1.9Hz, 1H), 6.93(d, J=7.5Hz, 2H), 7.56(d, J=7.5 Hz, 2H), 12.78(s, 1H);
[0039] (2) Take 15.7g of compound II, add 157mL of dimethyl sulfoxide with a solid-liquid ratio of 1:10, add 0.157g of potassium iodide, heat to 80°C, stir for 30h, and cool; we...
Embodiment 3
[0042] (1) Weigh 19.6g of 2,4-dimethoxy-6-hydroxyacetophenone, and the molar ratio of 2,4-dimethoxy-6-hydroxyacetophenone to p-methoxybenzaldehyde is 1 :1. Weigh 13.6g of p-methoxybenzaldehyde, then add 332mL of ethanol according to the solid-liquid ratio of 1:10 to dissolve, and then add potassium hydroxide to make the concentration of the alkali in the solution 25%, and stir at 30°C After 30h, the pH of the reaction solution was adjusted to 3 with concentrated hydrochloric acid solution, the reaction solution was suction filtered, the filter cake was washed with distilled water, and the filter cake was dried to obtain compound II; yield 88%; mp: 112~116; 1 HNMR (CDCl 3 ) Characterization: δ3.82(s, 3H), 3.85(s, 3H), 3.92(s,3H), 5.97(d,J=1.9Hz,1H), 6.11(d, J=1.9Hz, 1H), 6.93(d, J=7.5Hz, 2H), 7.56(d, J=7.5 Hz, 2H), 12.78(s, 1H);
[0043] (2) Take 15.7g compound II, add 78.5mL dimethyl sulfoxide according to the solid-liquid ratio of 1:5, add 0.471g sodium iodide, heat to 100°C, st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com