Substituted quinazolines
A compound and selected technology, which can be used in medical preparations containing active ingredients, extracellular fluid diseases, drug combinations, etc., can solve the problem of not realizing the anti-megakaryocyte potential, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
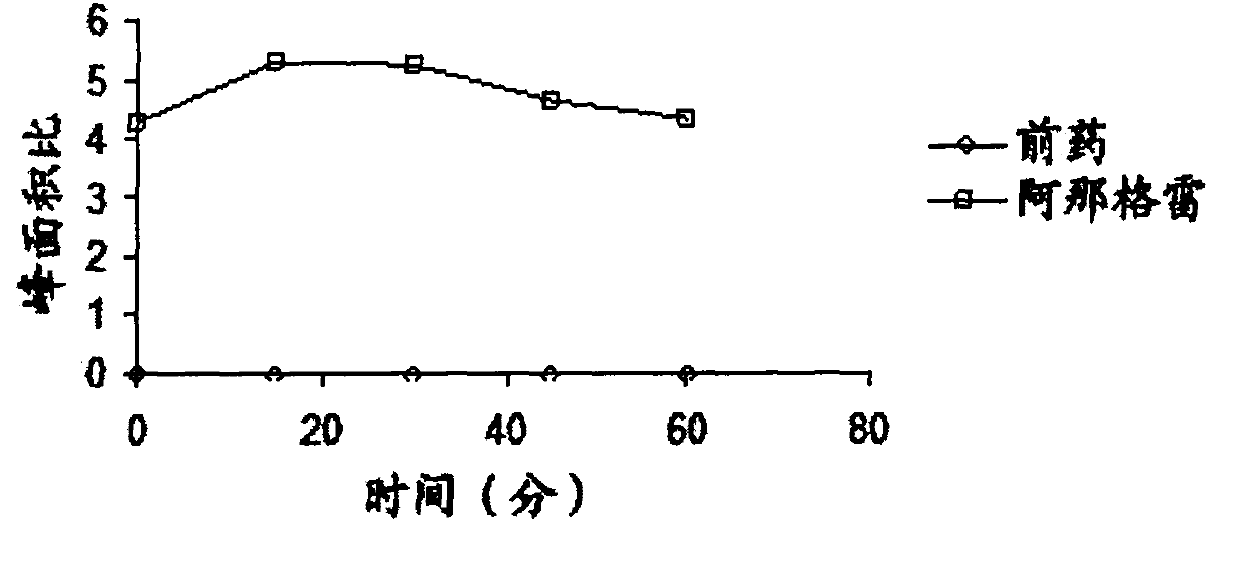
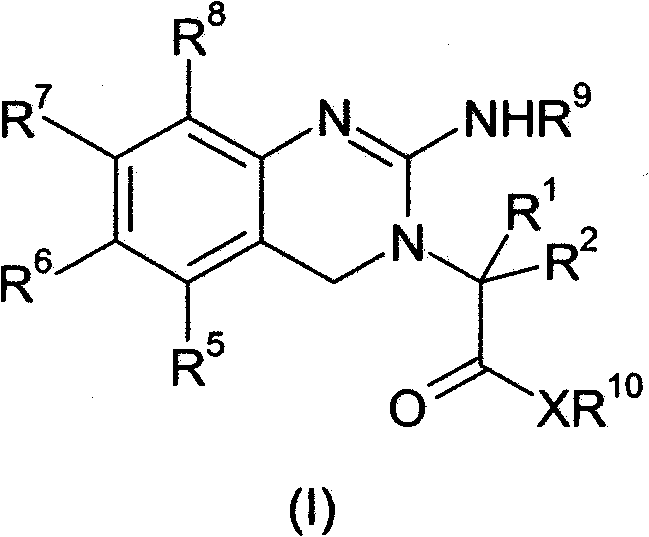
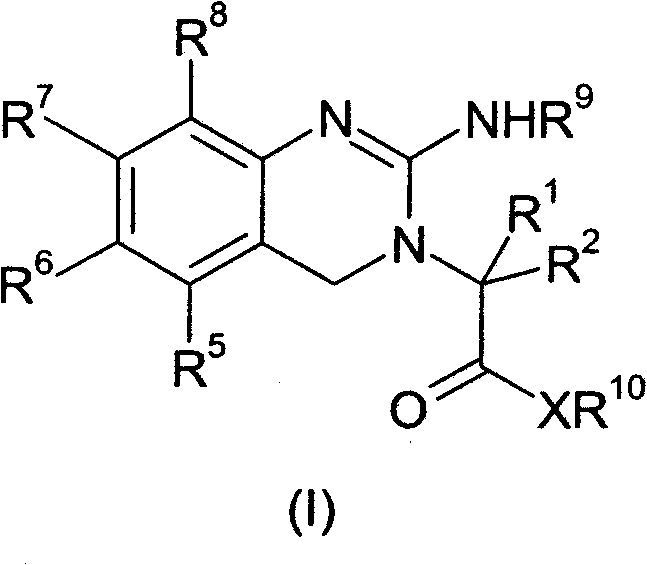
[0084] The present invention relates to novel prodrugs of substituted analogues of the established platelet reducing agent anagrelide. These compounds spontaneously ring-close at pH 7 and above to produce 3- or 5-substituted anagrelide, which retain the antimegakaryocyte properties of anagrelide (thus maintaining thrombocytopenic activity), but have reduced PDEIII inhibitory properties, and thus Has a lower potential for adverse cardiovascular and antiaggregative side effects.
[0085] Appropriate substitution at the 3-position of the anagrelide molecule effectively blocks the main metabolic site and thus prevents the formation of the highly potent PDE III inhibitor 3-OH anagrelide. 5-Substituted analogs have the potential to indirectly sterically interfere with metabolism at the preferred 3-position. These 3- or 5-substituted analogs of anagrelide also have the potential to improve the pharmacokinetic profile, since the 3-position of the anagrelide molecule is known to be th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com