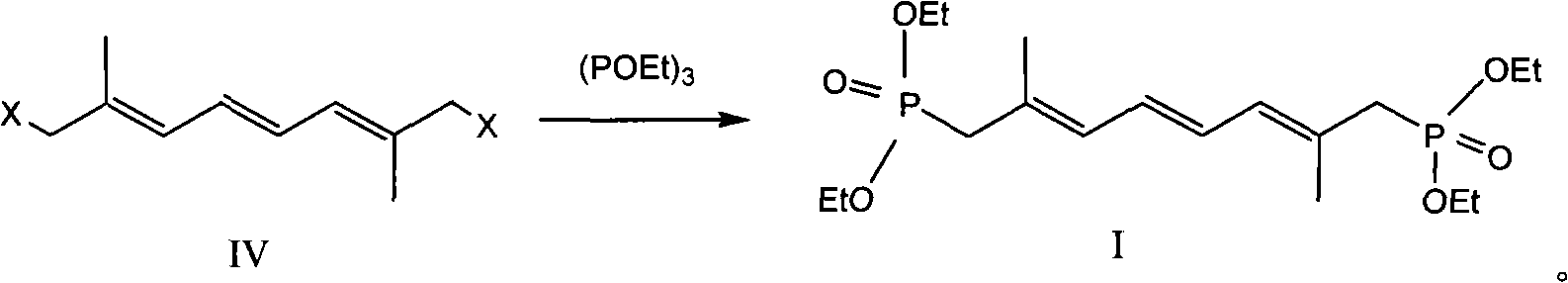
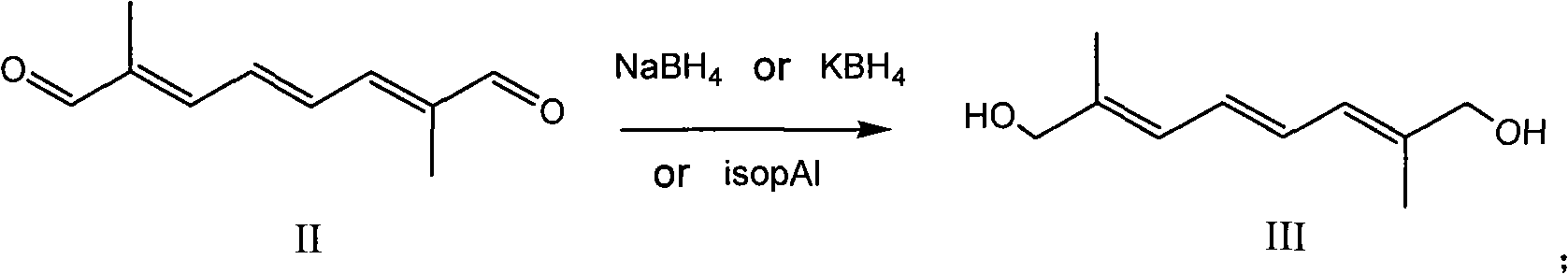Preparation method of 2,7-dimethyl-2,4,6-octa-triene-1,8-diethylester biphosphonate
A technology of diethyl diphosphonate and octatriene, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the difficulties in the recovery of the by-product triphenylphosphine oxide, Triphenylphosphine is highly toxic and difficult to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Put 8.2g (0.05mol) of 2,7-dimethyl-2,4,6-octatriene-1,8-dialdehyde (decadecandialdehyde) (II) and 100mL of isopropanol into a 500mL three-necked flask and stir Dissolve, control the temperature at 25-30°C, add 20.5g (0.1mol) of aluminum isopropoxide in batches, keep warm and reflux for 5h. After freezing again to 25-30°C, use 1N hydrochloric acid to adjust the pH to about 6, recover the solvent, add 200 mL of water and 100 mL of chloroform, dissolve the separated salt, separate the layers, extract the aqueous layer with 50 mL of chloroform, and combine the organic layers , recovered and dried to obtain 7.1 g of 2,7-dimethyl-2,4,6-octatriene-1,8-diol (III), with a yield of 84.5%.
[0034] 1 H NMR (CDCl 3 , 200MHz) δ: 6.51 (2H, m), 6.25 (2H, m), 4.20 (4H, m), 1.71 (2H, m).
Embodiment 2
[0036] Add 2,7-dimethyl-2,4,6-octatriene-1,8-dialdehyde (8.2 g (0.05 mol) of decabisaldehyde (II) and 100 mL of dimethyltetrahydrofuran into a 500 mL three-necked flask and stir Dissolve, cool to -5°C in an ice-salt bath, control the reaction temperature at 0-5°C, add 6.5g (0.12mol) of potassium borohydride in batches, control the temperature at 25-30°C after the injection, and keep warm for 4h. Freeze again until After 0~-5°C, use glacial acetic acid to adjust the pH to about 6, recover dimethyltetrahydrofuran, add 200mL of water and 100mL of dichloromethane, dissolve the separated salt, separate the layers, extract the aqueous layer with 50mL of dichloromethane, and combine the organic layer, recovered and dried to obtain 7.0 g of 2,7-dimethyl-2,4,6-octatriene-1,8-diol (III), with a yield of 83.4%.
[0037] 1 H NMR (CDCl 3 , 200MHz) δ: 6.51 (2H, m), 6.25 (2H, m), 4.20 (4H, m), 1.7l (2H, m).
Embodiment 3
[0039]Drop into 2,7-dimethyl-2,4,6-octatriene-1,8-dialdehyde (decadecandialdehyde (II) 8.2g (0.05mol) and ethanol 100mL in 500mL three-necked flask and stir to dissolve, ice Cool the salt bath to -5°C, control the reaction temperature at 0~-5°C, add 3.8g (0.1mol) of sodium borohydride in batches, control the temperature at 25~30°C after the injection, and keep it warm for 2~3h. Freeze again to 0 After ~-5°C, use glacial acetic acid to adjust the pH to about 6, recover ethanol, add 200 mL of water and 100 mL of dichloromethane to dissolve the separated salt, separate layers, extract the aqueous layer with 50 mL of dichloromethane, combine the organic layers, and recover to dryness. 7.5 g of 2,7-dimethyl-2,4,6-octatriene-1,8-diol III was obtained, with a yield of 89.3%.
[0040] 1 H NMR (CDCl 3 , 200MHz) δ: 6.51 (2H, m), 6.25 (2H, m), 4.20 (4H, m), 1.71 (2H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com