Method for producing polycrystalline silicon by utilizing sodium fluosilicate byproduct of phosphate fertilizer
A technology of sodium fluorosilicate and by-products, applied in the field of polysilicon production, can solve the problems of non-compliance with green environmental protection development, pollute the environment, and reduce the conversion rate of raw materials, and achieve the effects of saving raw materials, low impurity content, and reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The production method of the present embodiment is as follows:
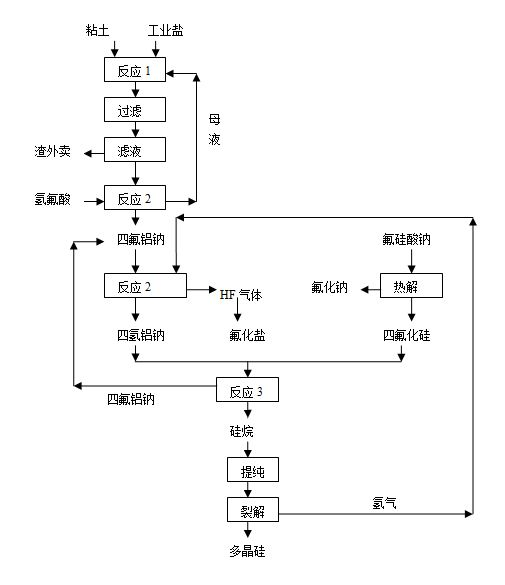
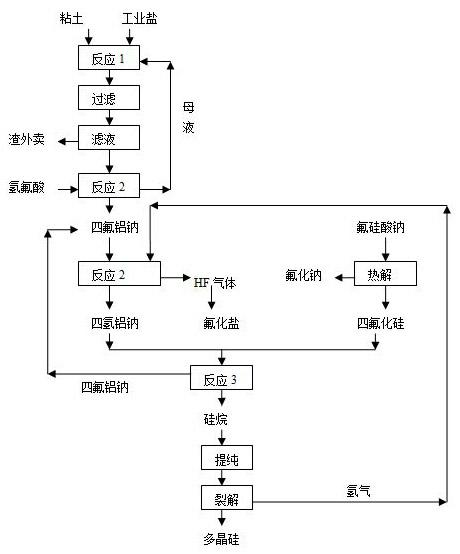
[0027] (1) Using clay, industrial salt, and hydrochloric acid as raw materials, leaching at 70°C for 0.5h according to the ratio of Na / Al=0.95, and then filtering, reacting the filtrate with hydrofluoric acid to produce sodium tetrafluoroaluminum, and returning the mother liquor ;
[0028] (2) React the aluminum tetrafluoroaluminum sodium prepared above with hydrogen according to the theoretical ratio at 600°C under the action of palladium catalyst to generate sodium tetrahydroaluminum;
[0029] (3) At the same time, pyrolyze sodium fluorosilicate, a by-product of phosphate fertilizer required by the theoretical ratio, at 700°C to generate sodium fluoride and silicon tetrafluoride gas;
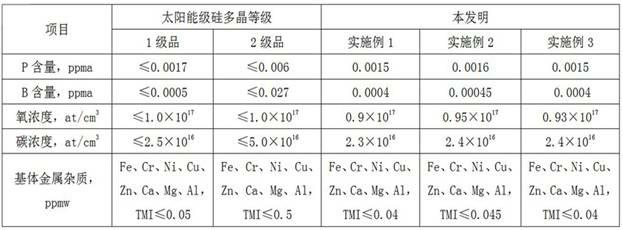
[0030] (4) React sodium aluminum tetrahydrogen and silicon tetrafluoride with a mass ratio of 2:1 in a mixed solvent of glycol dimethyl ether and toluene at 60°C to obtain silane and sodium tetrafluoroaluminum, sodium tet...
Embodiment 2
[0035] The production method of the present embodiment is as follows:
[0036] (1) Using clay, industrial salt, and hydrochloric acid as raw materials, leaching at 80°C for 1 hour according to the ratio of Na / Al=1.0, then filtering, reacting the filtrate with hydrofluoric acid to produce sodium tetrafluoroaluminum, and returning the mother liquor ;
[0037] (2) The sodium tetrafluoroaluminum sodium prepared above is reacted with hydrogen according to the theoretical ratio at 400°C under the action of a nickel catalyst to form sodium tetrahydroaluminum;
[0038] (3) At the same time, pyrolyze sodium fluorosilicate, a by-product of phosphate fertilizer required for the theoretical ratio, at 770°C to generate sodium fluoride and silicon tetrafluoride gas;
[0039] (4) React sodium aluminum tetrahydrogen and silicon tetrafluoride with a mass ratio of 3:1 in a mixed solvent of glycol dimethyl ether and toluene at 80°C to obtain silane and sodium tetrafluoroaluminum, aluminum tetra...
Embodiment 3
[0044] The production method of the present embodiment is as follows:
[0045] (1) Using clay, industrial salt, and hydrochloric acid as raw materials, leaching at 90°C for 1 hour according to the ratio of Na / Al=1.1, then filtering, reacting the filtrate with hydrofluoric acid to produce sodium tetrafluoroaluminum, and returning the mother liquor ;
[0046] (2) React the aluminum tetrafluoroaluminum sodium prepared above with hydrogen in a theoretical ratio at 200°C under the action of a nickel catalyst to form sodium tetrahydroaluminum;
[0047] (3) At the same time, pyrolyze sodium fluorosilicate, a by-product of phosphate fertilizer required by the theoretical ratio, at 900°C to generate sodium fluoride and silicon tetrafluoride gas;
[0048] (4) React sodium aluminum tetrahydrogen and silicon tetrafluoride with a mass ratio of 4:1 in a mixed solvent of dimethyl ether and toluene at 100°C to obtain silane, sodium tetrafluoroaluminum, and aluminum tetrafluoride Sodium recy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com