Method of producing 2'-deoxy-5-azacytidine (decitabine)
A technology of decitabine and azacytidine, which is applied in the field of thioalkyl derivatives to achieve the effects of easy removal, improved final yield and easy handling
Active Publication Date: 2011-04-27
CILAG
View PDF4 Cites 16 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Does not require the use of ester compounds as catalysts
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
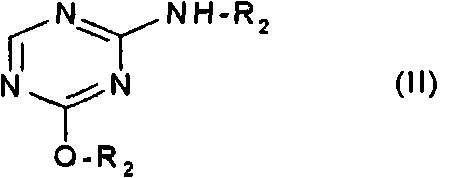
The invention relates to a method of producing 2'-deoxy-5-azacytidine (Decitabine) by providing a compound of formula (I) shown in the description, wherein R is a removable substituent known per se; and R1 is a removable substituent; further providing a silylated base of formula (II) shown in the description, wherein R2 is a protecting group, preferably a trimethylsilyl TMS )-residue; reacting the compound of formula (I) and the compound of formula (II) together in a suitable anhydrous solvent and in the presence of a suitable catalyst; and removing the substituents R from the compound obtained in order to obtain the compound 2'-deoxy-5-azacytidine (Decitabine), characterized in that said catalyst is selected from the group comprising a salt of an aliphatic sulphonic acid or a salt of a strong inorganic acid.
Description
technical field The present invention relates to a process for the production of 2'-deoxy-5-azacytidine (decitabine) by reacting a glycoside donor with a selected silylated base in the presence of a selected catalyst, The glycoside donor is preferably a 1-halogenated derivative of blocked mono-saccharide, or an imidate, preferably a trichloromethyl derivative, or a thioalkyl derivative. Background technique Decitabine is a nucleoside and is a known pharmaceutically active compound. It is known from US 3,817,980 that by silylation of the corresponding nucleoside base and combining the silylated base with a glycoside donor, preferably a 1-halogenated derivative of a capped monosaccharide, on a selected catalyst The reaction is carried out in the presence of nucleosides. The catalyst used is selected from e.g. SnCl 4 、TiCl 4 , ZnCl 2 , BF 3 - etherate (BF 3 -etherate), AlCl 3 and SbCl 5 . The main disadvantage is that these catalysts are prone to hydrolysis, producing...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07H19/12
CPCC07H19/12A61P31/12A61P35/00Y02P20/55
Inventor O·琼格曼N·克劳特
Owner CILAG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com