Application of sphaelactone and derivative thereof to treatment of cancers
A derivative, cancer technology, applied in the field of preparation of anti-cancer or auxiliary anti-cancer drugs, can solve the problems of drug resistance, tumor stem cell insensitivity, etc., and achieve the effect of strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
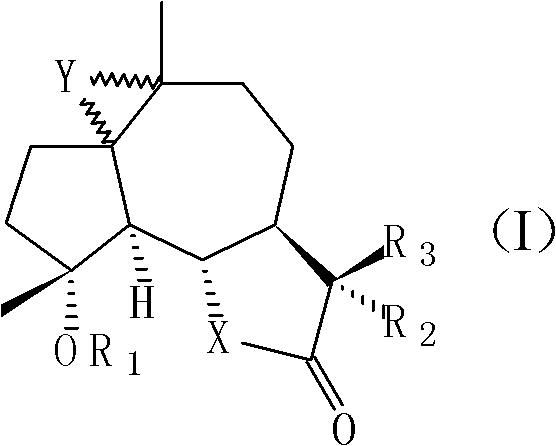
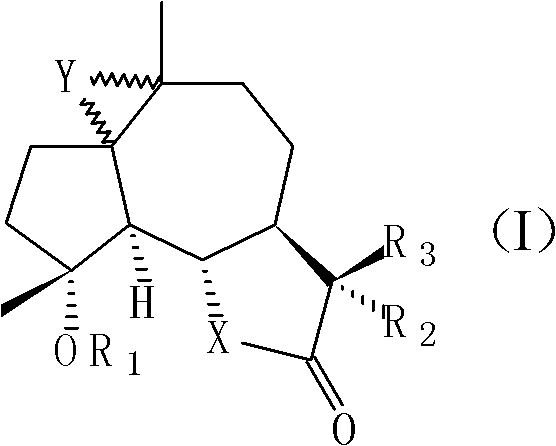
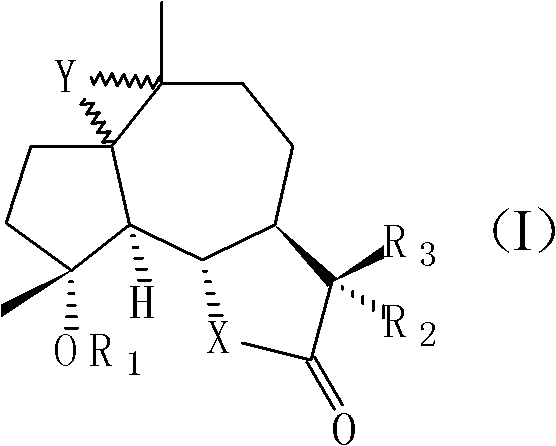
[0039] 11βH, the preparation of 13-dimethylaminomildolactone (compound 1, structural formula as following formula (II))
[0040]
[0041] Michelactone (106mg, 0.40mmol), triethylamine (2.0mL), and methanol (30mL) were added to a 100mL round-bottomed flask, heated to reflux for 3 hours, concentrated under reduced pressure, and silica gel column chromatography (petroleum ether: ethyl acetate Ester: triethylamine=50:50:0.5) to obtain 107.4 mg of white solid, yield: 86%.
[0042] Molecular formula: C 17 h 27 NO 3
[0043] Molecular weight: 293
[0044] Appearance: white amorphous powder
[0045] Spectral data:
[0046] 1 H NMR (CDCl 3 , 400MHz) δ3.76(t, J=10.0Hz, 1H), 2.96(s, 1H), 2.49-2.67(m, 3H), 2.28-2.34(m, 1H), 2.30-2.34(m, 2H) , 2.18(s, 6H), 2.09(brs, 2H), 1.96(d, J=11.2, 1H), 1.67-1.73(m, 2H), 1.60(s, 3H), 1.22(brs, 3H), 1.18 (br s, 2H); 13 C NMR (CDCl 3 , 100MHz) δ177.0, 131.8, 131.3, 84.0, 80.2, 58.3, 58.1, 50.9, 46.0, 44.6, 38.4, 35.3, 30.0, 27.2, 23.7, 22...
Embodiment 2
[0048] Preparation of 4-propionyl michelolactone (compound 2, structural formula as following formula (III))
[0049]
[0050] Michelolactone (106mg, 0.40mmol), triethylamine (2.0mL), propionyl chloride (0.2mL), and 5mL dichloromethane were added to a 20mL round-bottomed flask, stirred at room temperature for 24h, concentrated under reduced pressure, and silica gel column layer Analysis (petroleum ether: ethyl acetate = 90:10) gave 87 mg of white solid, yield: 72%. Structural data of the prepared 4-propionylmiglianolide:
[0051] Molecular formula: C 18 h 24 NO 4
[0052] Molecular weight: 304
[0053] Appearance: white amorphous powder
[0054] Spectral data:
[0055] 1 H NMR (CDCl 3 , 400MHz) δ6.14(s, 1H), 5.42(s, 1H), 3.74(t, J=10.0Hz, J=10.0Hz, 1H), 1.80-2.74(m, 12H), 1.67(s, 3H ), 1.50(s, 3H), 1.07(t, J=4.0Hz, 3H); 13 C NMR (CDCl 3 , 100MHz) δ173.8, 170.1, 139.5, 131.5, 130.4, 118.6, 88.4, 83.0, 56.6, 50.1, 36.5, 34.9, 30.4, 28.7, 25.9, 24.1, 18.8, 9.1.
Embodiment 3
[0057] 1, the synthetic method of 1,10-epoxy michelolactone (compound 3, structural formula following formula (IV)):
[0058]
[0059] Michelactone (106mg, 0.40mmol), m-chloroperoxybenzoic acid (0.45mmol), and 5mL of dichloromethane were added to a 20mL round-bottomed flask, stirred at room temperature for 6h, concentrated under reduced pressure, and silica gel column chromatography (petroleum ether : ethyl acetate=80:20), to obtain 96 mg of white solid, yield: 91%. Structural data of the prepared 1,10-epoxy michelolactone:
[0060] Molecular formula: C 15 h 20 NO 4
[0061] Molecular weight: 264
[0062] Appearance: white amorphous powder
[0063] Spectral data:
[0064] 1 H NMR (CDCl 3 , 400MHz) δ6.13(d, J=3.2Hz, 1H), 5.44(d, J=2.8Hz, 1H), 3.73(t, J=10.4Hz, 1H), 1.30-2.46(m, 11H), 1.29(s, 3H), 1.28(s, 3H); 13 C NMR (CDCl 3 , 100MHz) δ168.7, 137.8, 118.6, 79.2, 77.3, 74.2, 66.7, 52.6, 48.4, 37.1, 33.8, 29.0, 24.6, 22.5, 21.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com