Preparation of novel anti-EGFR human source antibody MIL27 and application thereof
A technology of human antibodies and antibodies, which is applied in the direction of anti-animal/human immunoglobulins, antibodies, anti-receptors/cell surface antigens/cell surface determinant immunoglobulins, etc., which can solve the problem of limiting the use of anti-EGFR monoclonal antibodies , high cost, and insufficient clinical application of anti-EGFR monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Human Anti-EGFR Antibody Obtained by Computer Aided Molecular Design
[0040] 1. Materials:
[0041] InsightII 2005 program package (MSI molecular simulation, San Diego), which includes:
[0042] Hornology homology modeling;
[0043] Discover mechanical optimization;
[0044] Discover 3 room temperature dynamics simulation;
[0045] Docking molecular docking;
[0046] Delphi apparent electrostatic potential energy analysis.
[0047] Ludi molecular design program (1995);
[0048] IBM graphics workstation;
[0049] PDB 2008 database (download from www.rcsb.org);
[0050] SwissProt database (2008, online download);
[0051] Kabat database (2001, online download);
[0052] IMGT database (2008, online download).
[0053] 2. Method results:
[0054] Using the determined structural features of the functional epitope in the extracellular region of EGFR, a short peptide sequence that can recognize the characteristic epitope was designed by the Ludi program ...
Embodiment 2
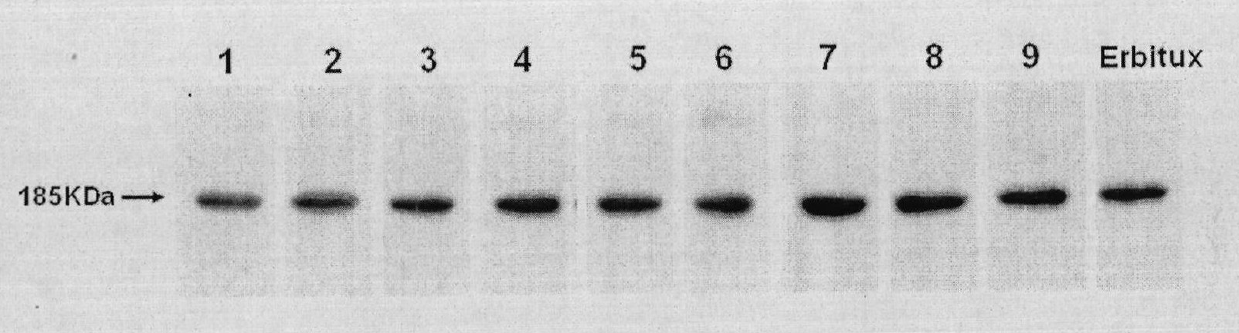
[0057] Example 2. Expression and Identification of Anti-EGFR Human Antibody MIL27
[0058] 1. Materials:
[0059] The primer design software was biosun software, and the primers were synthesized by Shanghai Yingjun Biotechnology Co., Ltd.; Pyrobest DNA polymerase was a product of TAKARA Company; dNTP was a product of TAKARA Company; pGEM-T Easy vector system was a product of Invitrogen Company; T4DNA ligase was a product of NEB Company ;Gene sequencing was completed by Beijing Nuosai Genome Research Center Co., Ltd.; the antibody eukaryotic expression vector pTGS-FRT-DHFR was constructed by our company and applied for a national patent (patent authorization number: ZL200510064335.0); liposome and MTT are products of Invitrogen , goat anti-human IgG, horseradish-labeled goat anti-human IgG and human IgG were prepared by our company; endonuclease was a product of NEB Company; other reagents were commercially available.
[0060] 2. Method results
[0061] 1. Synthesis of EGFR a...
Embodiment 3
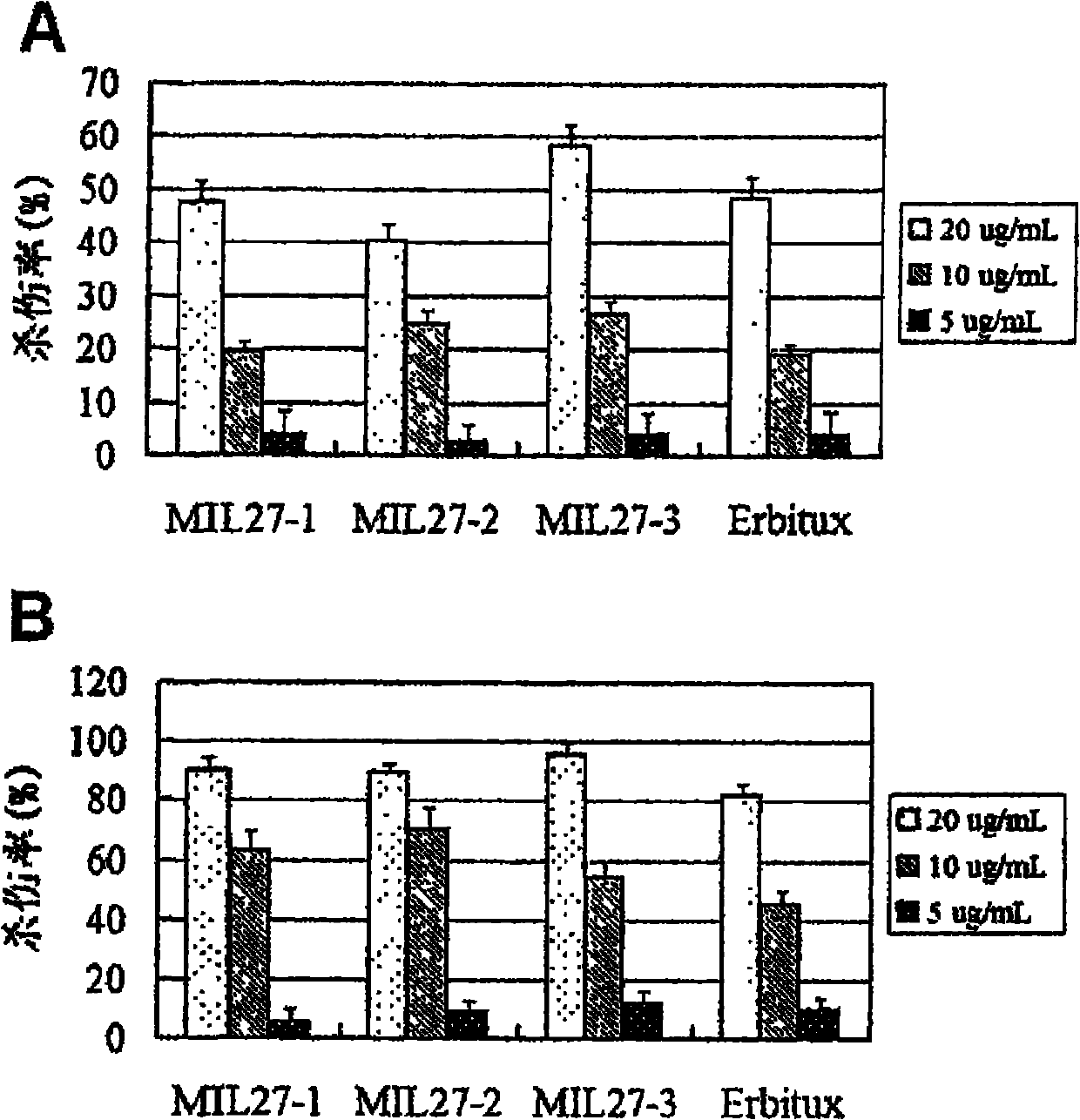
[0071] Example 3. Functional test of anti-EGFR antibody MIL27
[0072] EGFR human antibodies MIL27-1, MIL27-2 and MIL27-3 were taken as examples to further verify the biological functions of the antibodies.
[0073] 1. Materials
[0074] The breast cancer cell line SKVO3 and the liver cancer cell line HepG2 were purchased from ATCC; the DELFIAEuTDA cytotoxicity kit was a product of PE Company; other related reagents refer to Example 2.
[0075] 2. Method results
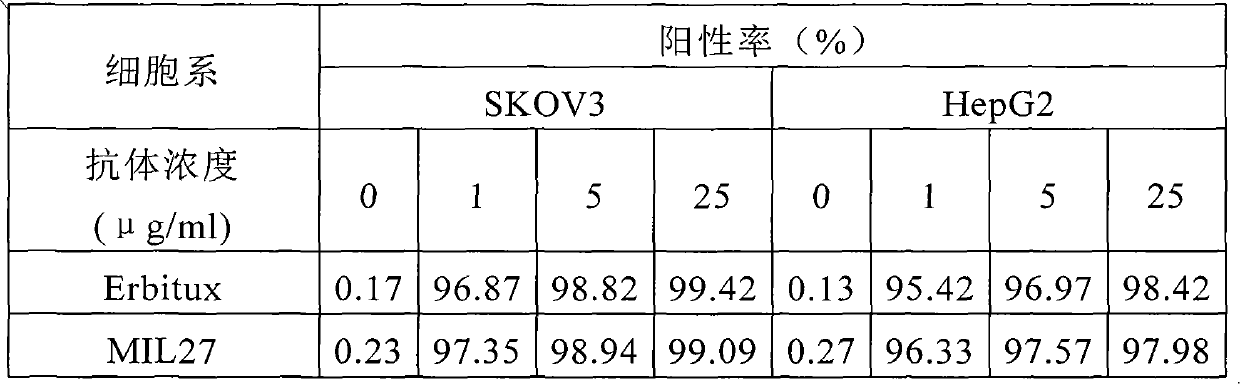
[0076] 1. Flow cytometry analysis of the binding of MIL27 to cell surface EGFR
[0077] EGFR-positive breast cancer cell line SKVO3, liver cancer cell line HepG2 and lung cancer cell line A549 were used as target cells to detect the ability of different concentrations of MIL27 antibodies to bind to cell surface EGFR antigens; Erbitux antibody was used as a positive control. The results showed that MIL27 can specifically recognize target cells, and its binding positive rate is similar to that of the control antibod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com