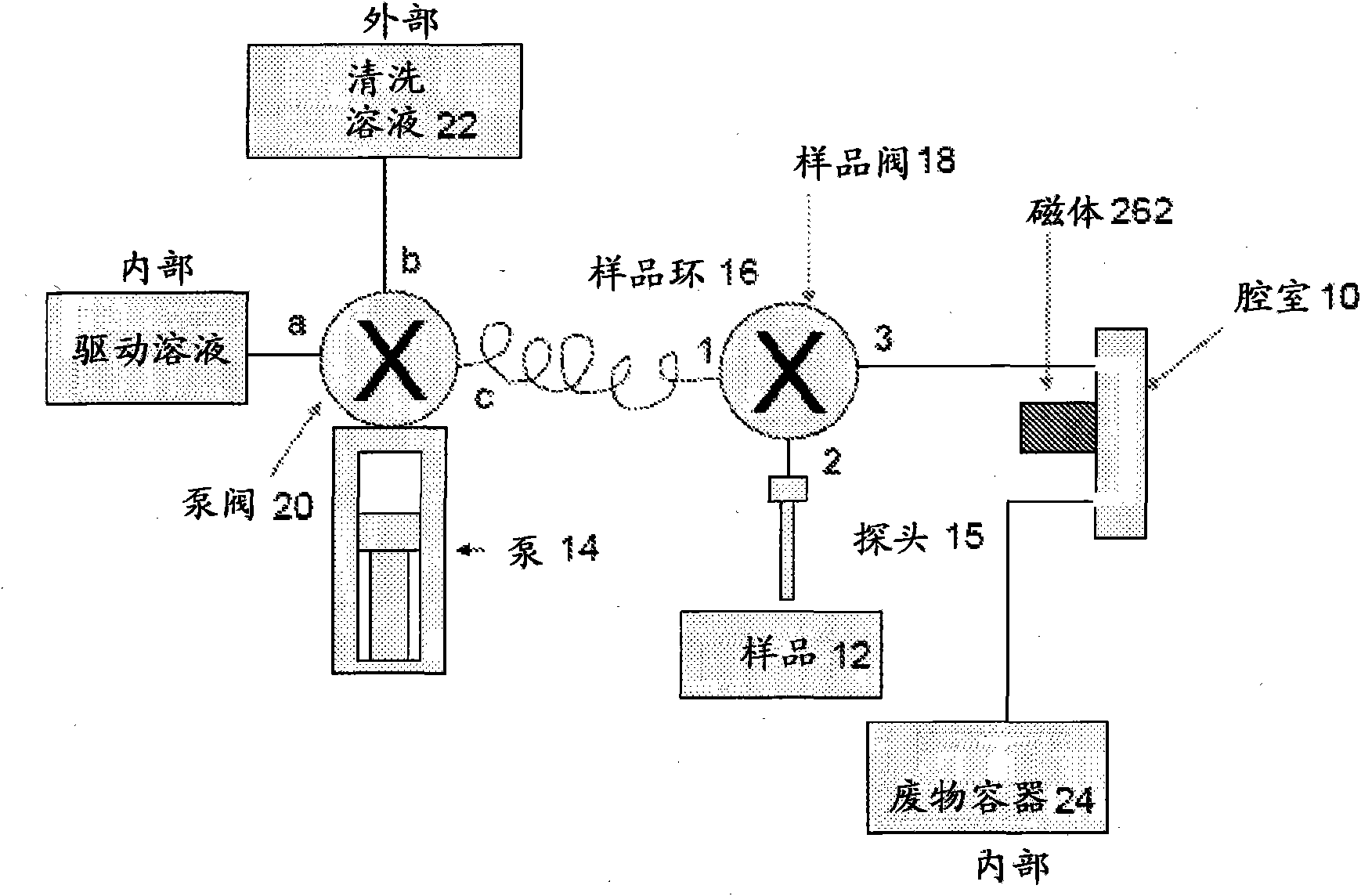
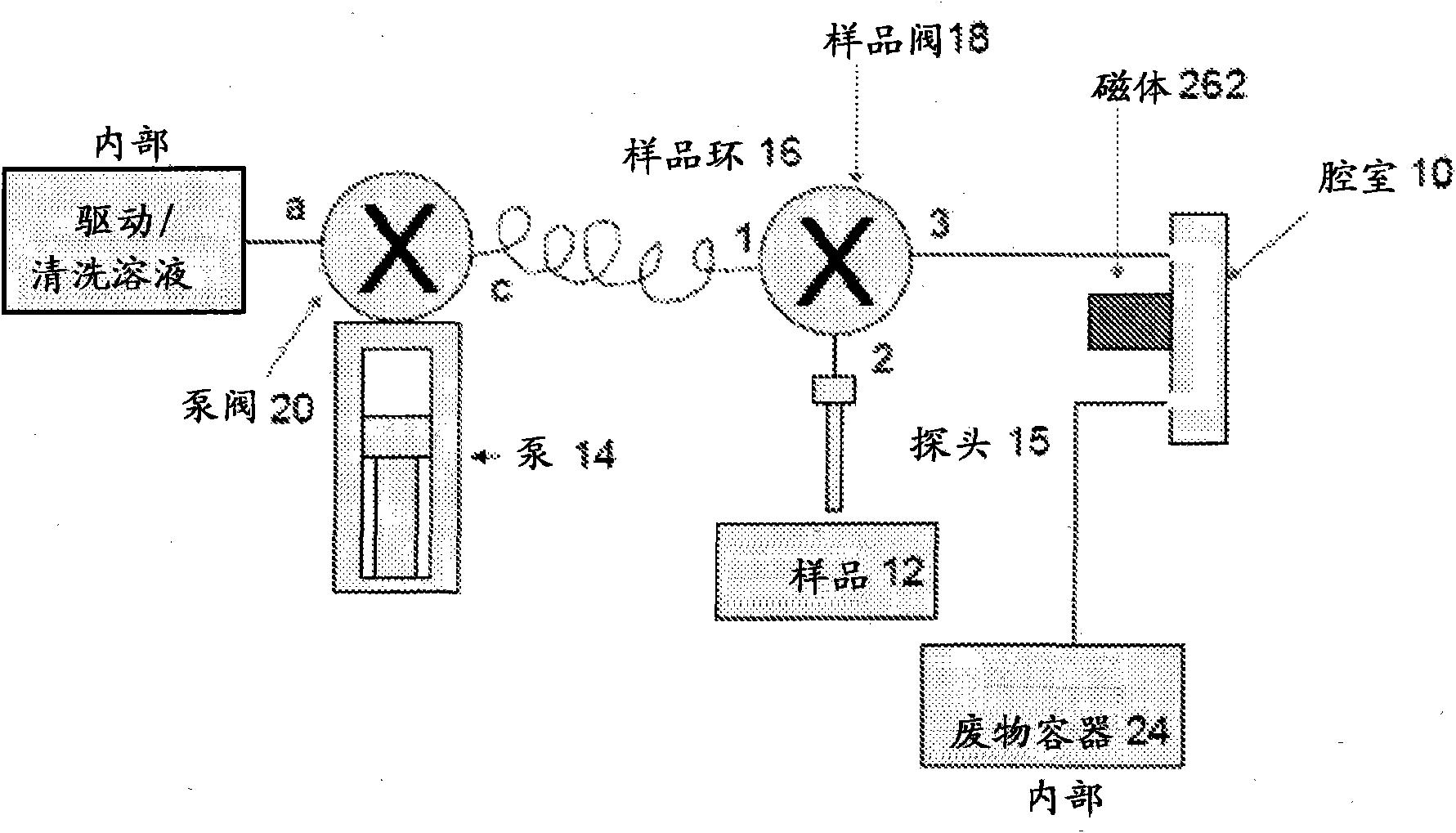
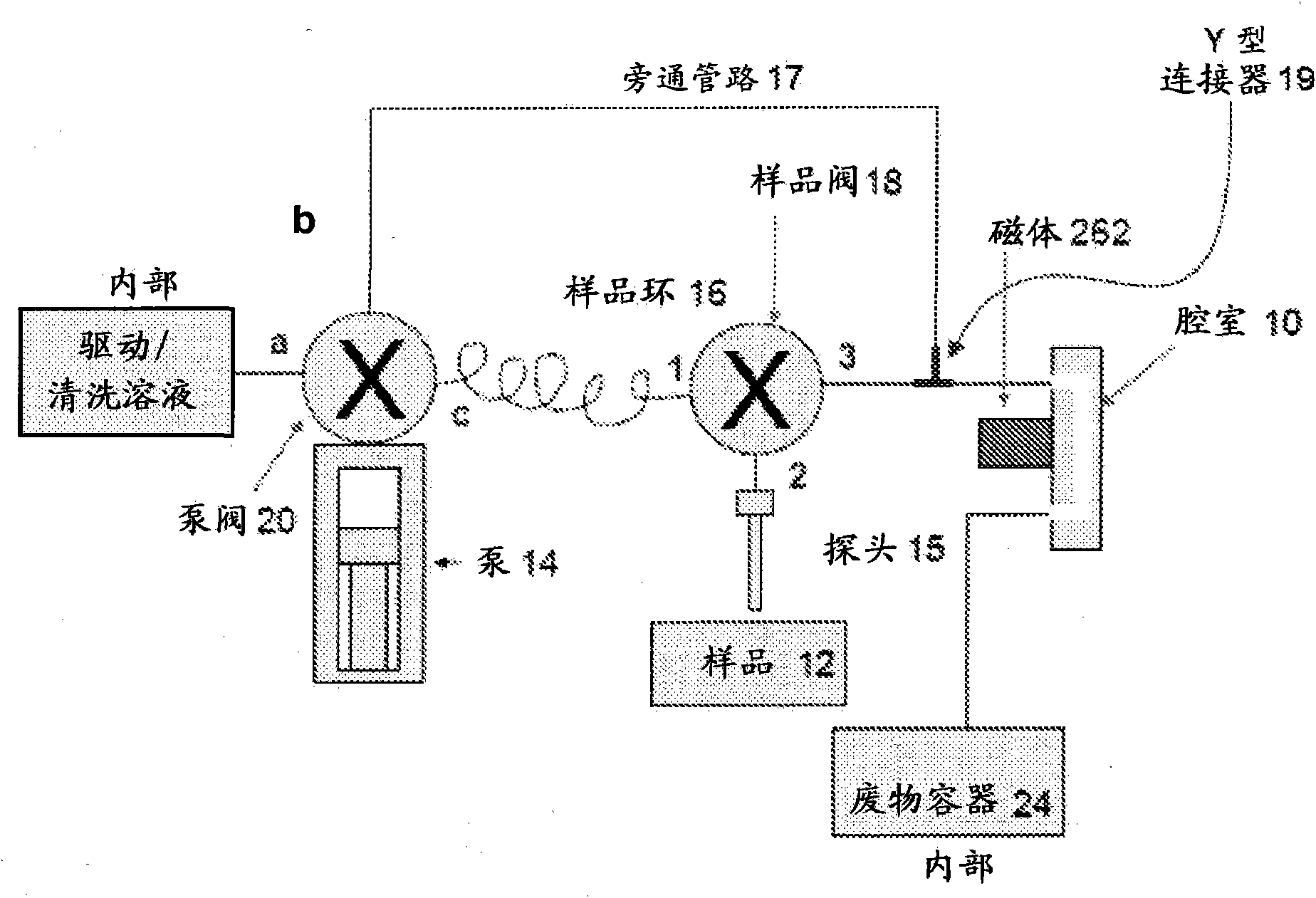
Immunomagnetic capture and imaging of biological targets
A magnetic, labeling technique used in immunomagnetic separation and fluorescence detection of such biological analytes, immobilization and detection of biological targets in samples, immobilization and detection of one or more biological target groups and/or subgroups in samples, capable of solve long-term problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0414] Example 1. CD4 cell count
[0415] In this example, the magnetic capture antibody is an anti-CD4 antibody, which captures all CD4 lymphocytes as well as all CD4 monocytes. The detection antibody is the same antibody or another anti-CD4 antibody conjugated with a fluorescent label. The real-time analysis process monitors the CD4 cell population in the imaging chamber. At the end of the sample loading step, all CD4 cells counted correspond to all CD4 lymphocytes and CD4 monocytes captured by the capture antibody and labeled by the detection antibody.
[0416] Whole blood samples were collected and stored in a BD Vacutainer (BD Biosciences #366452) containing 2 mM EDTA as an anticoagulant. Pipette 20 µL of sample into a 1.5 mL centrifuge tube. 5 μL magnetic anti-CD4 capture antibody (IMag TM Anti-human CD4 particles - BD Biosciences, Cat. No. 557767) were added directly to centrifuge tubes containing 20 μL of blood samples. Add 5 μL of phycoerythrin (PE)-labeled anti-...
Embodiment 2
[0418] Example 2. Enumeration of CD4 Lymphocytes by Subtraction Strategy
[0419] In this example, the magnetic capture antibody is an anti-CD4 antibody, which captures all CD4 lymphocytes as well as all CD4 monocytes. The detection antibody mixture contained anti-CD4 antibody conjugated to one fluorescent marker (Marker 1) and anti-CD14 antibody conjugated to another fluorescent marker (Marker 2). The real-time analysis process monitors the CD4 cell population in the imaging chamber. At the end of the sample loading step, all CD4 cells counted correspond to all CD4 lymphocytes and CD4 monocytes in the sample captured by the capture antibody. All CD14 cells counted corresponded only to the monocyte population in the sample captured by the capture antibody. CD4 lymphocyte counts were calculated as monocyte counts subtracted from total CD4 counts.
[0420] Whole blood samples were collected and stored in a BD Vacutainer (BD Biosciences #366452) containing 2 mM EDTA as an anti...
Embodiment 3
[0422] Example 3. Measuring Clinically Relevant Counts of CD3 Cells, CD4 Cells, and CD8 Cells
[0423] In this example, the magnetic capture antibody is an anti-CD3 antibody, which captures all lymphocytes. The detection antibody mix consisted of an anti-CD3 antibody conjugated to one fluorescent marker (Marker 1), an anti-CD4 antibody conjugated to another fluorescent marker (Marker 2), and a third fluorescent marker ( Marker 3) anti-CD8 antibody. The real-time analysis process monitors the CD3 cell population in the imaging chamber. Figure 7 Schematic of the field of view of the imaging system using the "Marker 1" detection channel, which detects the CD3 population (ie all lymphocytes) at time points T1, T2, ... Tn and Tfinal). Detection and counting of captured cells is repeated until the number of cells counted by the imaging processing algorithm exceeds a certain threshold. Once the number of cells in the field of view reaches the defined threshold, the sample loading...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com