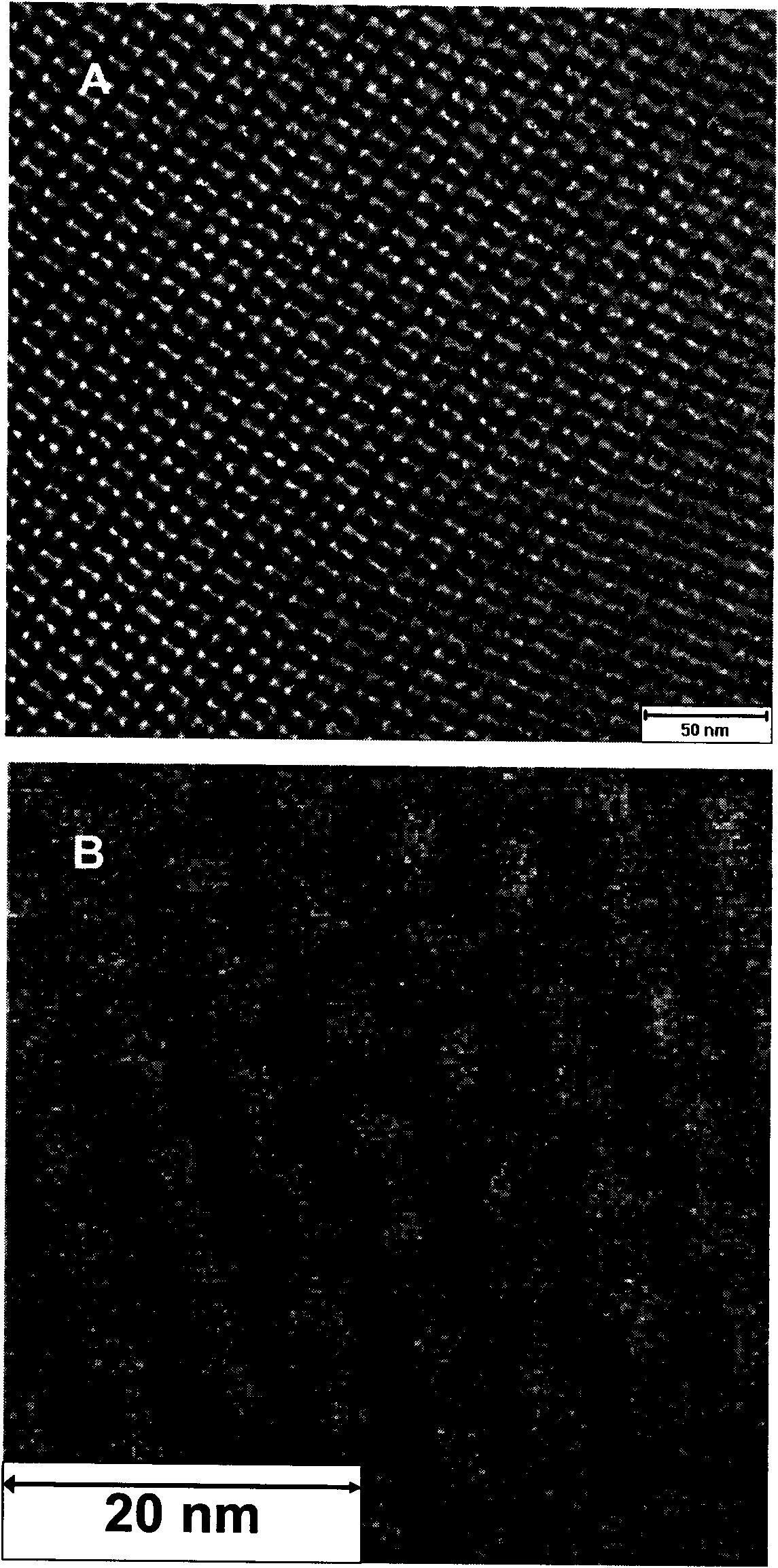
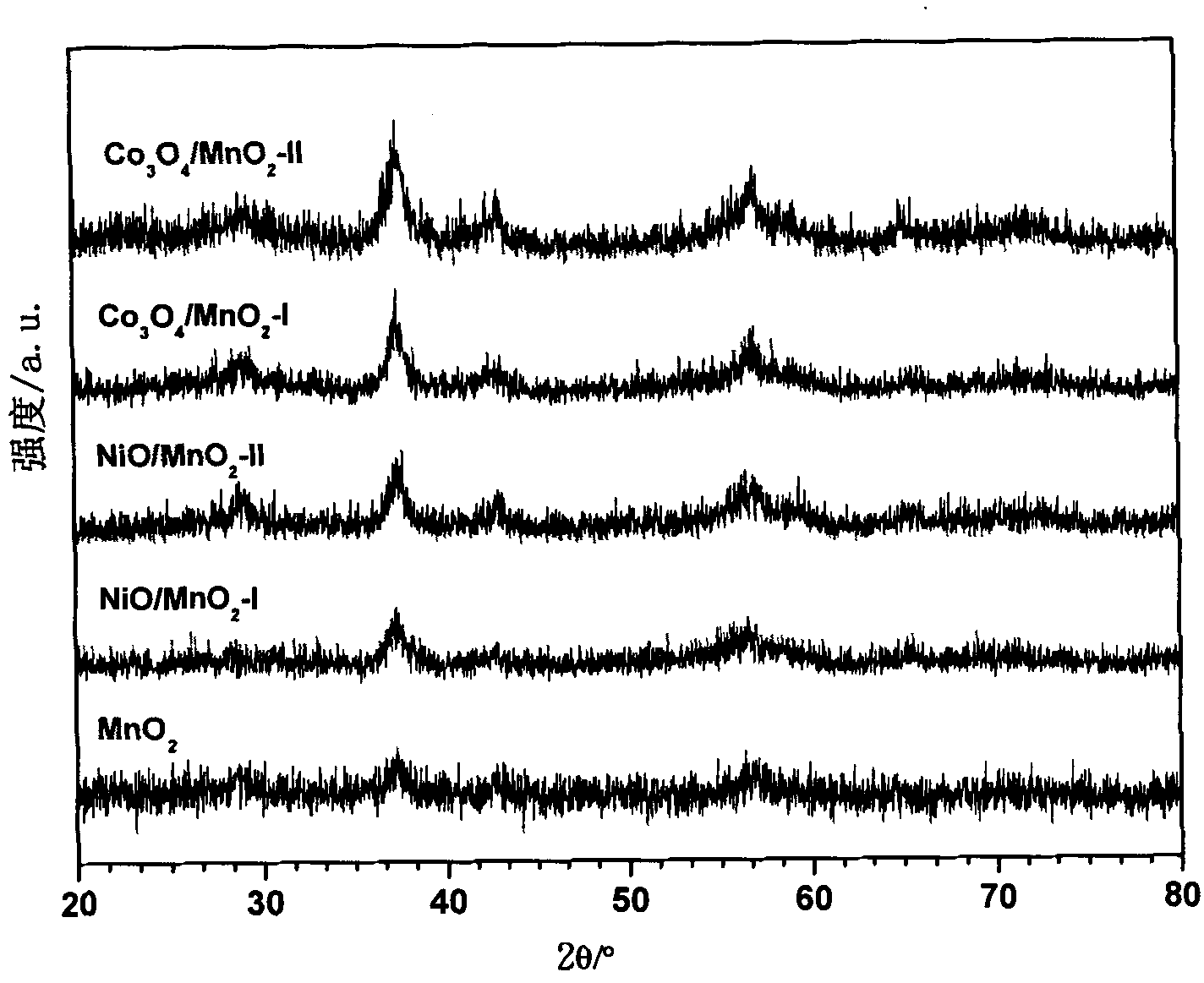
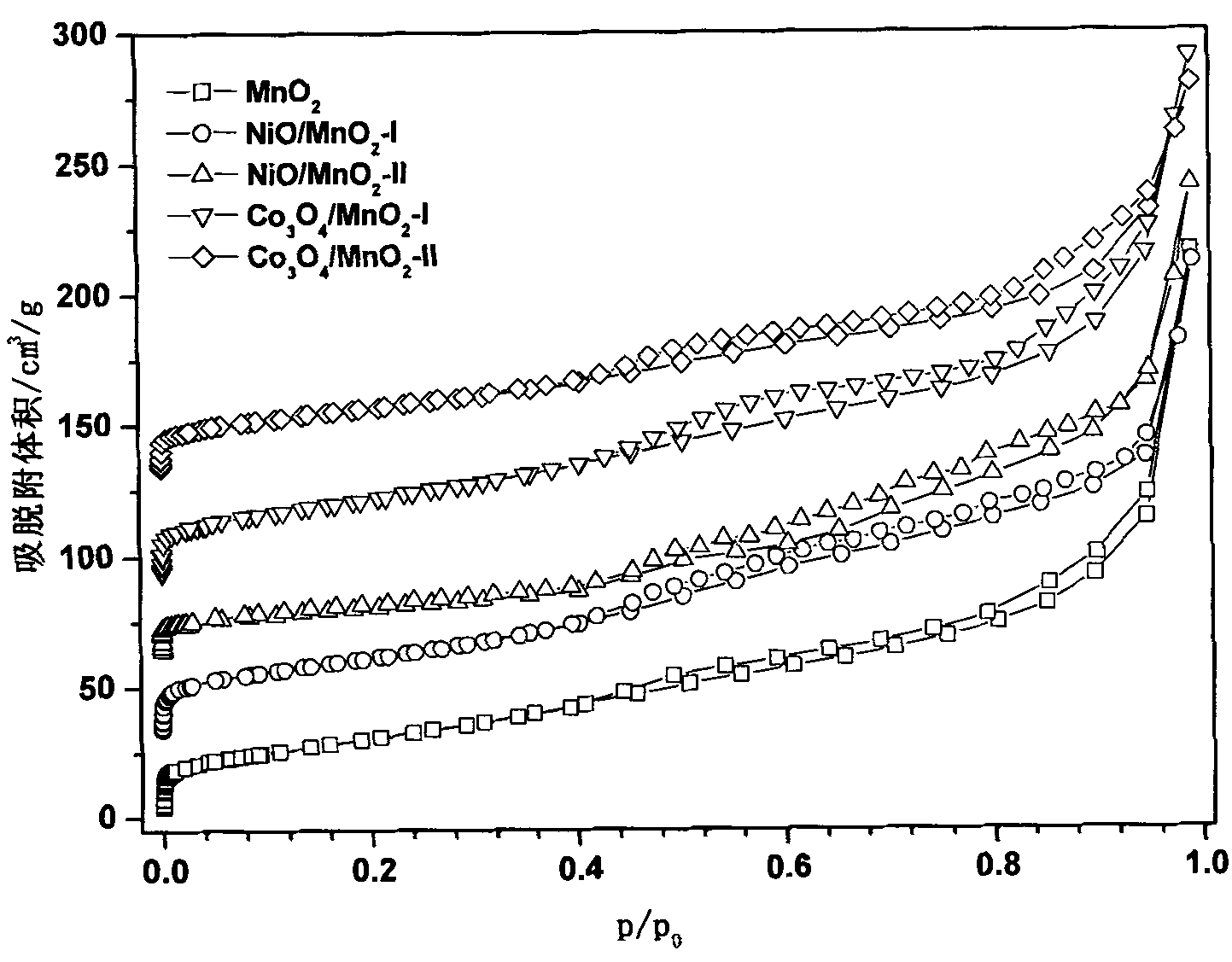
Preparation method and application of mesoporous Co3O4/ Beta-MnO2 or NiO/ Beta-MnO2 catalysts
A catalyst and mesoporous technology, applied in the field of transition metal composite catalysts and their preparation, to achieve the effects of low energy consumption, mild reaction conditions and economical benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Mn(NO 3 ) 2 4H 2 O and Co(NO 3 ) 2 ·6H 2O was dissolved in deionized water to form a 7 mol / L mixed solution, wherein the molar ratio of Mn to Co was 15:1. Add KIT-6 into n-hexane solution to form a 20g / L solution, and stir well at room temperature for 2 hours. In the case of stirring, add the above mixed solution of manganese nitrate and cobalt nitrate into the KIT-6 n-hexane solution, wherein the mass ratio of Mn to KIT-6 is 1.0:1, and then stir the mixed solution system at room temperature for 10 hours , suction filtration, and drying at 25°C to obtain a black product, which was placed in a tube furnace, purged with an air flow of 200mL / min, raised to 300°C at a rate of 1°C / min, and kept at this temperature for calcination for 2 hours. A black powder was obtained, which was washed with 0.001mol / L sodium hydroxide solution at 50°C to remove the KIT-6 template, washed with deionized water to remove residual impurities, and finally, the product was dried at 120...
Embodiment 2
[0029] (1) Mn(NO 3 ) 2 4H 2 O and Co(NO 3 ) 2 ·6H 2 O was dissolved in deionized water to form a 9 mol / L mixed solution, in which the molar ratio of Mn to Co was 35:1. Add KIT-6 into n-hexane solution to form a 30g / L solution, and stir thoroughly at room temperature for 4 hours. In the case of stirring, add the above mixed solution of manganese nitrate and nickel nitrate into the n-hexane solution of KIT-6, wherein the mass ratio of Mn to KIT-6 is 3.0:1, and then stir the mixed solution system at room temperature After 14 hours, filter with suction and dry at 40°C to obtain a black product, which is placed in a tube furnace, purged with an air flow rate of 300mL / min, heated to 400°C at 3°C / min, and calcined at this temperature for 4 hours. hours, a black powder was obtained, and then the black powder was washed with 0.009mol / L sodium hydroxide solution at 70°C to remove the KIT-6 template, washed with deionized water to remove residual impurities, and finally, the produc...
Embodiment 3
[0034] (1) Mn(NO 3 ) 2 4H 2 O and Ni(NO 3 ) 2 ·6H 2 O was dissolved in deionized water to form a 7 mol / L mixed solution, wherein the molar ratio of Mn to Ni was 16:1. Add KIT-6 into n-hexane solution to form a 20g / L solution, and stir well at room temperature for 2 hours. In the case of stirring, the above mixed solution of manganese nitrate and nickel nitrate was added to the KIT-6 n-hexane solution, wherein the mass ratio of Mn to KIT-6 was 1.0:1, and then the mixed solution system was stirred at room temperature for 10 hours, suction filtration, and drying at 25°C to obtain a black product, which was placed in a tube furnace, purged with an air flow of 200mL / min, raised to 300°C at a rate of 1°C / min, and kept at this temperature for calcination for 2 hours , to obtain a black powder, wash the black powder with 0.001mol / L sodium hydroxide solution at 50°C to remove the KIT-6 template, wash with deionized water to remove residual impurities, and finally, dry the product...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com