Method for preparing chitosan nanoparticles
A technology of chitosan nanoparticles and chitosan, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problems of poor clinical compliance and achieve good biological Compatibility, high drug loading, uniform distribution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
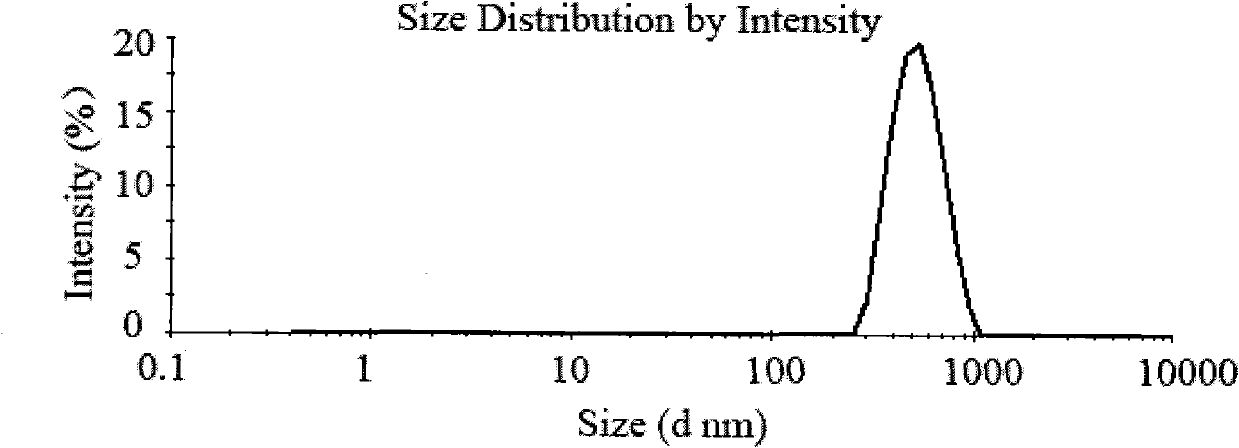
[0030] Weigh 500 mg of chitosan with a molecular weight of 50,000 Daltons and dissolve it in 50 mL of 1% acetic acid solution to obtain a 10 mg / mL chitosan solution. Prepare 20 mL of 5 mg / mL ammonium sulfate solution with distilled water. Slowly add ammonium sulfate solution dropwise under magnetic stirring, react for 30 minutes, sonicate the probe at 100W power for 2 minutes, centrifuge the above solution at 4 degrees Celsius and 10,000 rpm for 30 minutes to separate nanoparticles. Gained nanoparticles were redispersed in 50 mL of water to obtain chitosan nanoparticles. Use nano-ZS90 Malvern particle size analyzer to measure the particle size of nanoparticles, the average particle size is 826nm, the particle size distribution see figure 1 .
Embodiment 2
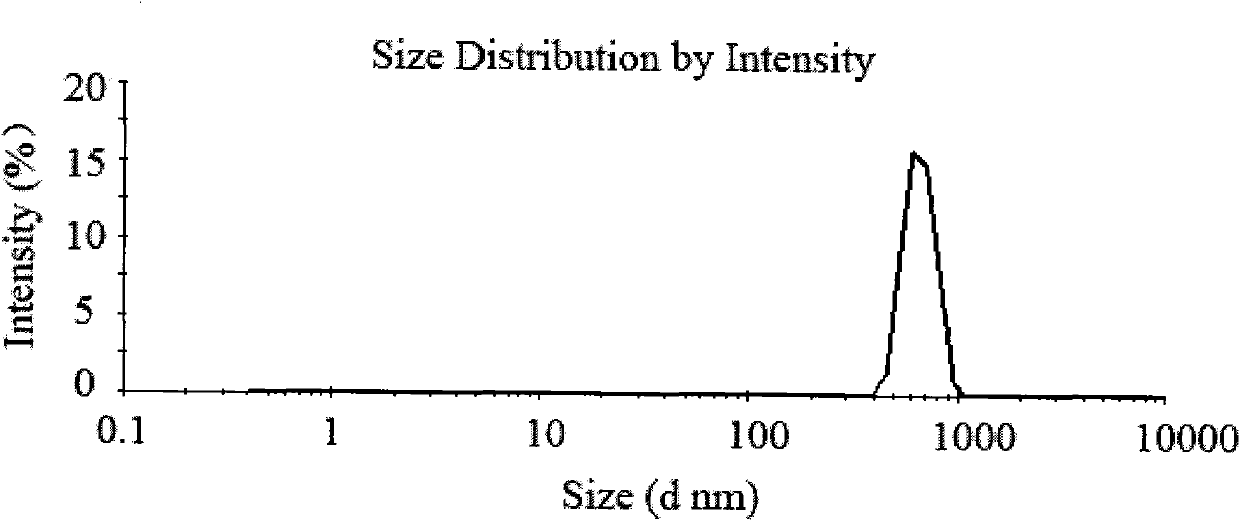
[0032] Weigh 200 mg of chitosan with a molecular weight of 100,000 Daltons and dissolve it in 20 mL of 1% acetic acid solution to obtain a 10 mg / mL chitosan solution. Prepare 20 mL of 5 mg / mL sodium sulfate solution with distilled water. Add 100mg of Endostar acetate buffer solution (pH5.5) into the chitosan solution, slowly add sodium sulfate solution dropwise under magnetic stirring, react for 10 minutes, ultrasonicate the probe for 2 minutes under 100W power, and put the above solution at 4 degrees Celsius, 15000 Centrifuge at rpm for 30 minutes to separate the nanoparticles. Gained nanoparticles were redispersed in 30 mL of water to obtain chitosan nanoparticles.
[0033] The supernatant solution after centrifugation was appropriately diluted, and the BCA method was used (the kit was from ThermoScientific, named BCA TM Protein Assay Kit) was used to measure the protein concentration, that is, the free protein concentration, and the weight percentage of recombinant human...
Embodiment 3
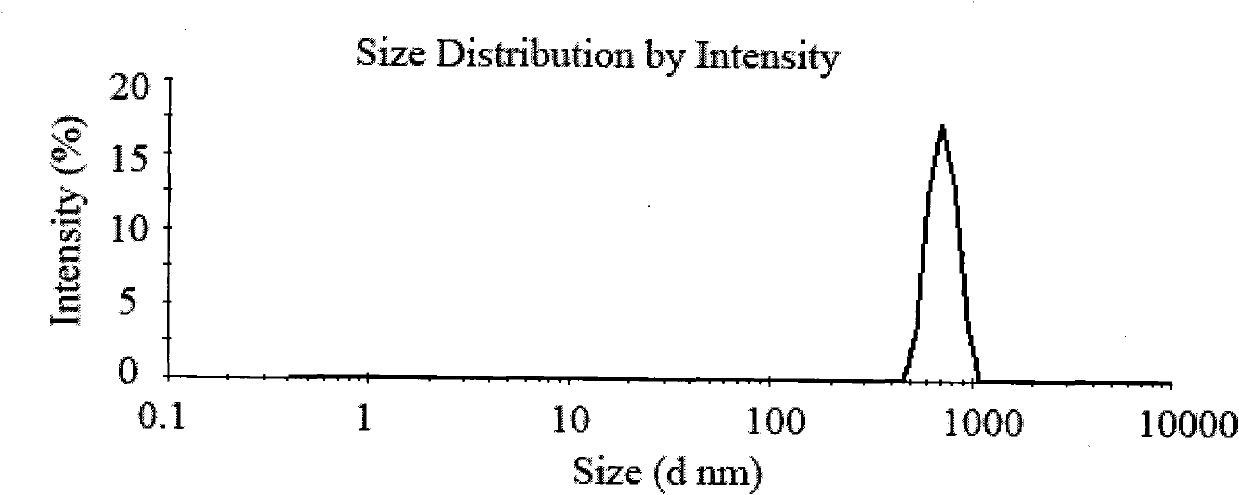
[0035] Weigh 200 mg of chitosan with a molecular weight of 100,000 Daltons, dissolve it in 40 mL of 1% acetic acid solution to obtain a 5 mg / mL chitosan solution, and add 200 μL of Tween 80 to dissolve it. Prepare 40 mL of 2 mg / mL sodium sulfate solution with distilled water. Take 100mg of Endostar acetate buffer solution (pH5.5) and add it to the chitosan solution containing Tween, slowly add sodium sulfate solution dropwise under magnetic stirring, react for 20 minutes, and ultrasonicate the probe for 2 minutes under 100W power, and dissolve the above solution Centrifuge at 15,000 rpm for 30 minutes at 4°C to separate nanoparticles. Gained nanoparticles were redispersed in 50 mL of water to obtain chitosan nanoparticles.
[0036] The determination of drug loading is the same as in Example 2. The particle size of the nanoparticle solution was measured with a nano-ZS90 Malvern particle size analyzer. The drug loading of the obtained nanoparticles is 20.1%, the average parti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com