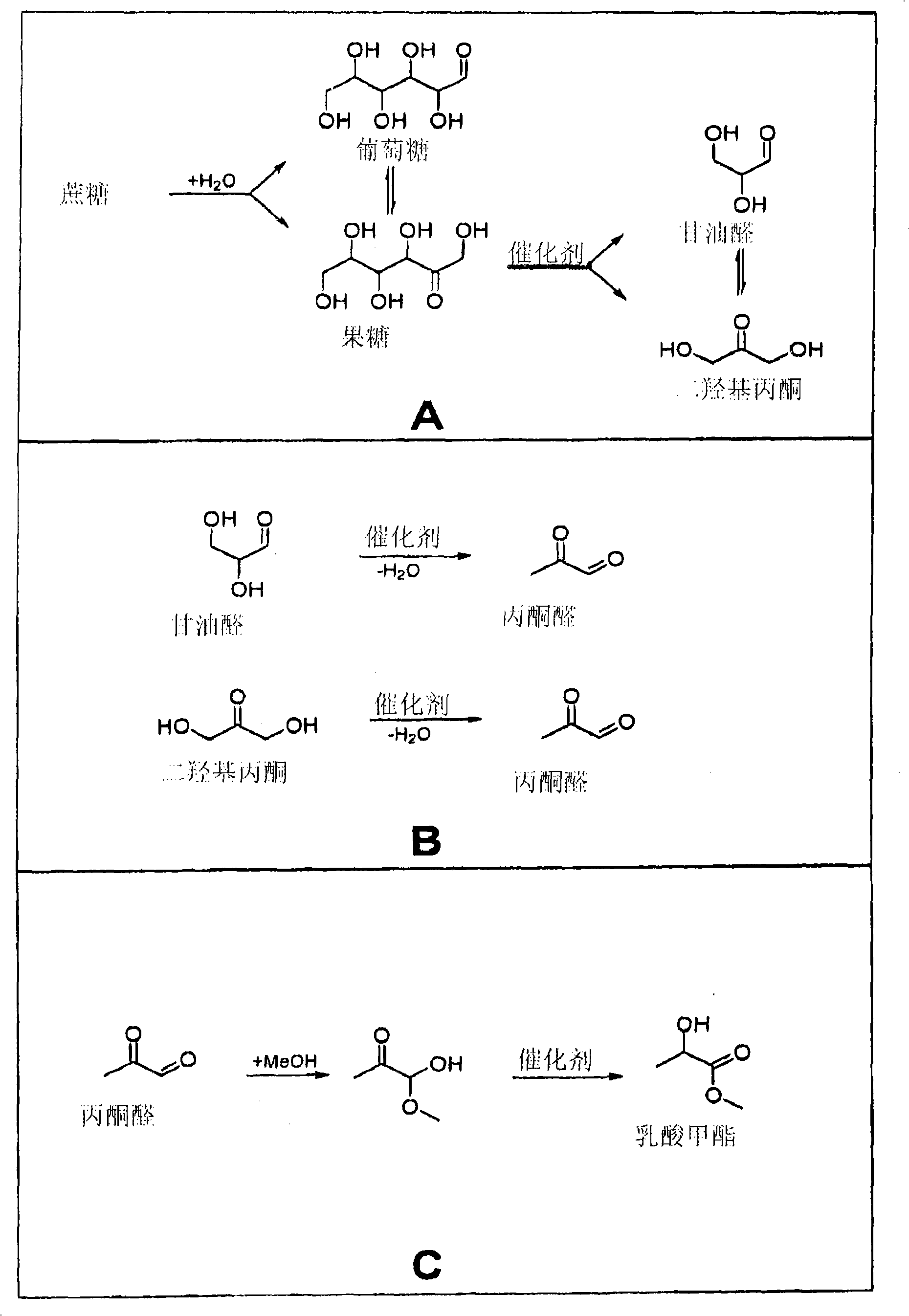
Zeolite-catalyzed preparation of alpha-hydroxy carboxylic acid compounds and esters thereof
A hydroxyl and catalyst technology, applied in the field of α-hydroxycarboxylic acid compounds, can solve problems such as large energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 8.0g of methanol, 0.2250g of sucrose (0.6576mmol), 121.3mg of naphthalene (internal standard) and finally 160.2mg of Sn-BEA (prepared according to US Patent 6.306.364) into an autoclave (50cc miniature autoclave). The autoclave was closed, filled with 20 bar argon and heated to 160°C. When the temperature reached 100° C., the mechanical stirring was started (500 rpm) and the mixture was heated under these conditions for 20 h. GC analysis of the reaction mixture showed the formation of 1.74 mmol methyl lactate (66%) and 0.022 mmol methyl 2-hydroxy-3-butenoate (1%).
Embodiment 2
[0018] Add 8.0g of methanol, 0.2251g of glucose (1.250mmol), 119.3mg of naphthalene (internal standard) and finally 160.3mg of Sn-BEA (prepared according to US Patent 6.306.364) into an autoclave (50cc miniature autoclave). The autoclave was closed, filled with 20 bar argon and heated to 160°C. When the temperature reached 100° C., the mechanical stirring was started (500 rpm) and the mixture was heated under these conditions for 20 h. GC analysis of the reaction mixture showed the formation of 1.02 mmol methyl lactate (41%) and 0.051 mmol methyl 2-hydroxy-3-butenoate (3%).
Embodiment 3
[0020] Add 8.0g methanol, 0.2251g fructose (1.250mmol), 120.0mg naphthalene (internal standard) and finally add 162.0mg Sn-BEA (prepared according to US Patent 6.306.364) in an autoclave (50cc miniature autoclave). The autoclave was closed, filled with 20 bar argon and heated to 160°C. When the temperature reached 100° C., the mechanical stirring was started (500 rpm) and the mixture was heated under these conditions for 20 h. GC analysis of the reaction mixture showed the formation of 1.07 mmol methyl lactate (43%) and 0.068 mmol methyl 2-hydroxy-3-butenoate (4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com