Spherical manganese dioxide type lithium ionic sieve
A manganese dioxide type, lithium ion technology, applied in other chemical processes, chemical instruments and methods, etc., can solve the problems of difficult post-processing, difficult application, and easy bed collapse.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
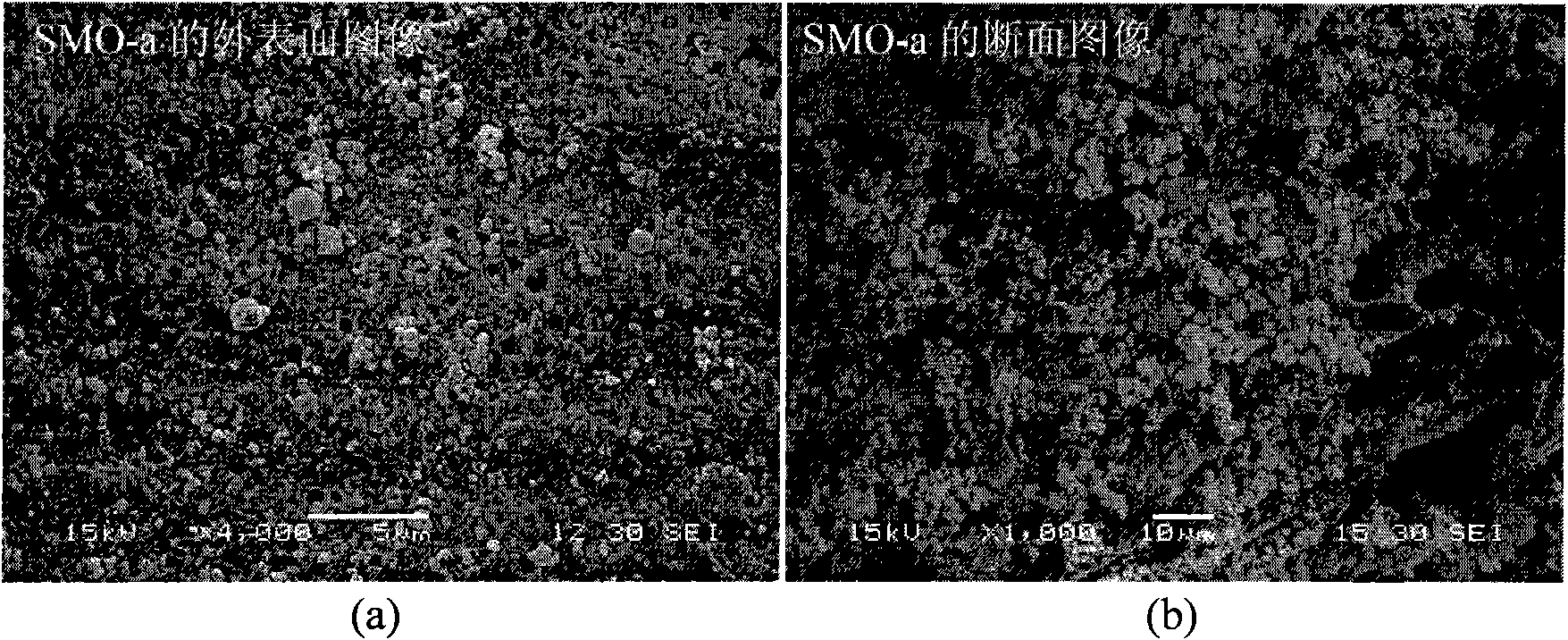
[0034] Under the conditions of 5℃~50℃ and normal pressure (1atm), weigh 4.00g of polyvinyl chloride (polymerization degree: 1000±20) and dissolve it in 66ml of NMP. After the dissolution is uniform, add 20.00g of LiMn 2 o 4 Ultrafine powder, after stirring evenly, drop into 400ml deionized water with a dropper with a diameter of 3mm, wash, and dry at 40-180°C for 10-30 hours to obtain spherical LiMn 2 o 4 type ion sieve precursor. The resulting spherical LiMn 2 o 4 placed in 0.5mol·l -1 Soak in hydrochloric acid (H / Li=1.5, molar ratio) for 24 hours, wash with deionized water, and dry at 40-180°C for 10-30 hours to obtain spherical λ-MnO 2 (LiMn 2 o 4 The H type) is recorded as SMO-a, and the specific surface area (BET) of SMO-a is: 13.02m 2 g -1 , SEM images of the outer surface and cross-section of SMO-a are shown in figure 1 .
Embodiment 2
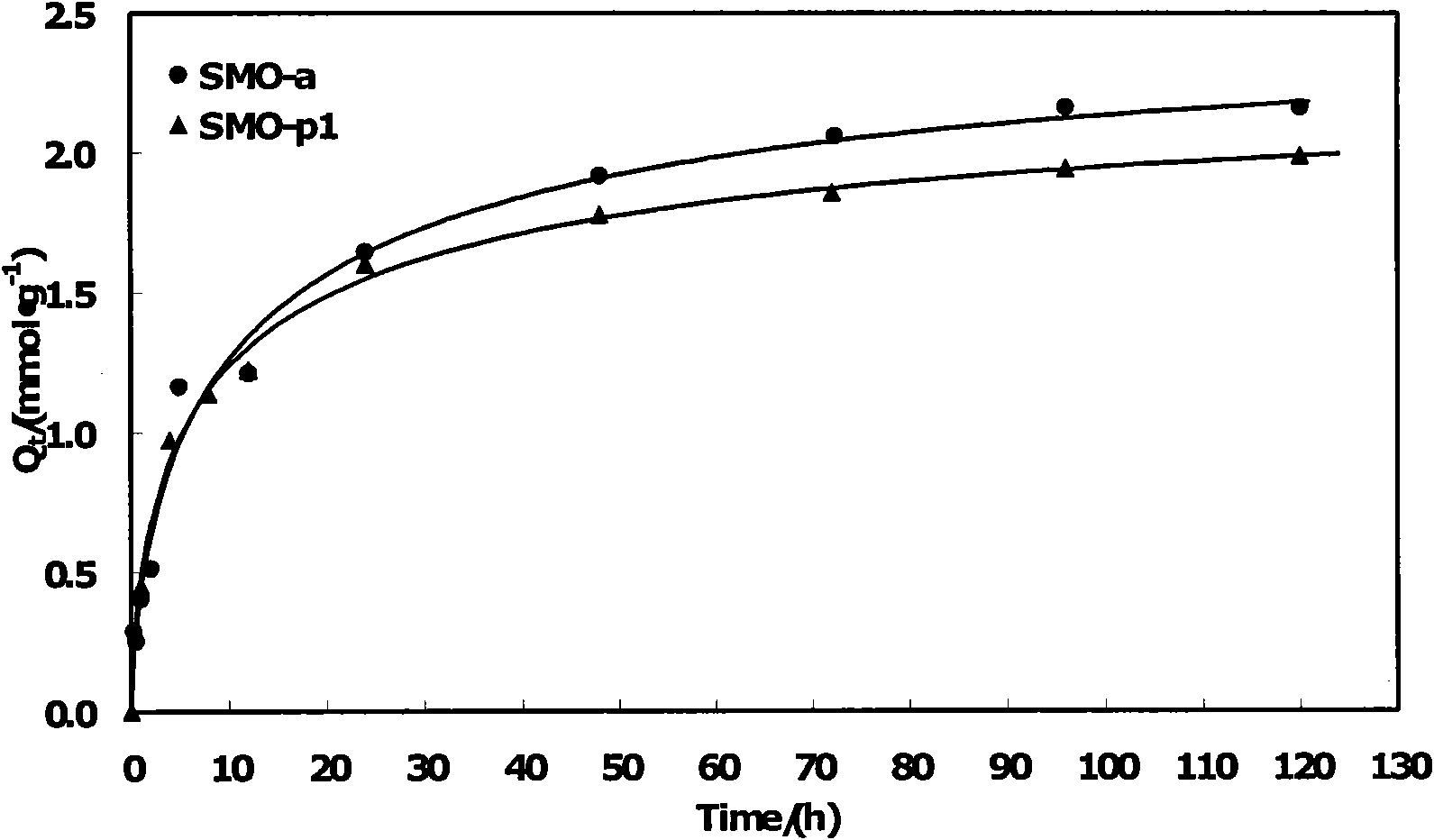
[0036]Weigh a series of quantitative SMO-a granular lithium ion sieves prepared in Example 1 and the corresponding powder ion sieve SMO-p1 respectively, and measure 0.010mol l -1 LiCl NH 3 ·H 2 O-NH 4 Cl buffer solution (pH value 9-11), mix the two and put them into a constant temperature oscillator for adsorption experiments. The adsorption temperature is 30°C and the rotation speed is 150r min -1 , respectively measure the concentration of lithium ions in the solution at different times. The adsorption capacity of SMO-a is 2.19mmol·g -1 , corresponding to the existing ultrafine powder ion sieve (marked as SMO-p1), the adsorption capacity of SMO-p1 is 1.99mmol·g -1 . The experimental results show that the performance of the spherical ion sieve SMO-a is better than that of the powder SMO-p1. The results show that the use of PVC bonded molding has little effect on the lithium adsorption performance of ion sieves.
[0037] see results figure 2 .
Embodiment 3
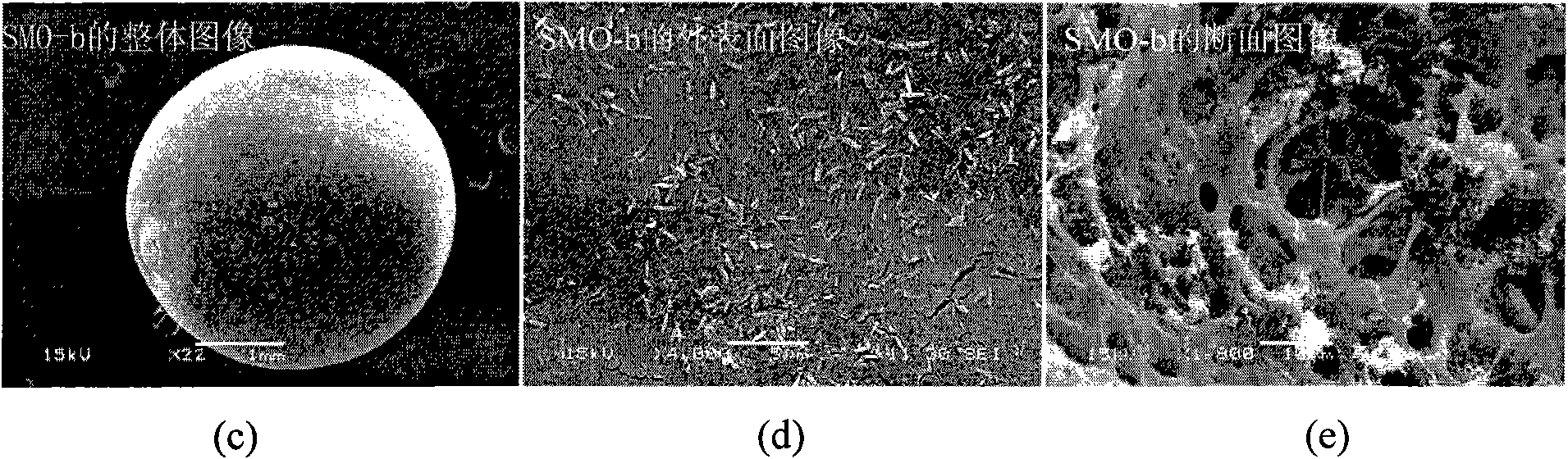
[0039] Under the conditions of 5℃~50℃ and normal pressure (1atm), weigh 6.00g of polyvinyl chloride (polymerization degree: 800±10) and dissolve it in 66ml of NMP, and add 20.00g of Li 4 mn 5 o 12 Super powder, after stirring evenly, use a dropper with a diameter of 3mm to drop into a mixed solution of 400ml deionized water and ethanol, wash, and dry at 40-180°C for 10-30 hours to obtain spherical Li4Mn 5 o 12 type ion sieve precursor. The resulting spherical Li 4 mn 5 o 12 Put in 1.0mol·l -1 Soak in hydrochloric acid (H / Li=4, molar ratio) for 24 hours, wash with deionized water, and dry at 40-180°C for 10-30 hours to obtain spherical MnO 2 Ion sieve (Li 4 mn 5 o 12 Type H) is denoted as SMO-b. The specific surface area (BET) of SMO-b is: 36.32m 2 g -1 , whose SEM images are shown in image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com