Chemically modified electrode, preparation thereof and method for rapid determination of acid value of plant oil
A technology of chemical modification and vegetable oleic acid, which is applied in the field of chemically modified electrodes and its preparation and rapid determination of vegetable oil acid value, achieving the effects of good stability and repeatability, excellent sensitivity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A chemically modified electrode for measuring the acid value of vegetable oil is a platinum electrode modified by polypyrrole doped with perchlorate ions. Electropolymerization of pyrrole polymers doped with perchlorate ions on the surface of platinum electrodes.
[0042] The preparation method of the chemically modified electrode is as follows: it is prepared by electrochemical polymerization, the surface of the platinum electrode is cleaned and dried for later use, and the cleaned and dried platinum electrode is placed in a modification solution, which contains pyrrole monomer and tetrabutyl An acetonitrile solution of ammonium perchlorate, the concentration of pyrrole monomer in the modification solution is 0.015mol / L, and the concentration of perchlorate anion is 0.1mol / L. Before electropolymerization, the modification solution is treated with nitrogen to remove the Then use the electrochemical workstation to carry out electropolymerization using constant potential ...
Embodiment 2
[0045] This example is an experiment conducted to determine the stability and reproducibility of polypyrrole-doped tetrabutyl perchlorate ammonium modified electrode for evaluation.
[0046] a. Evaluation of reproducibility
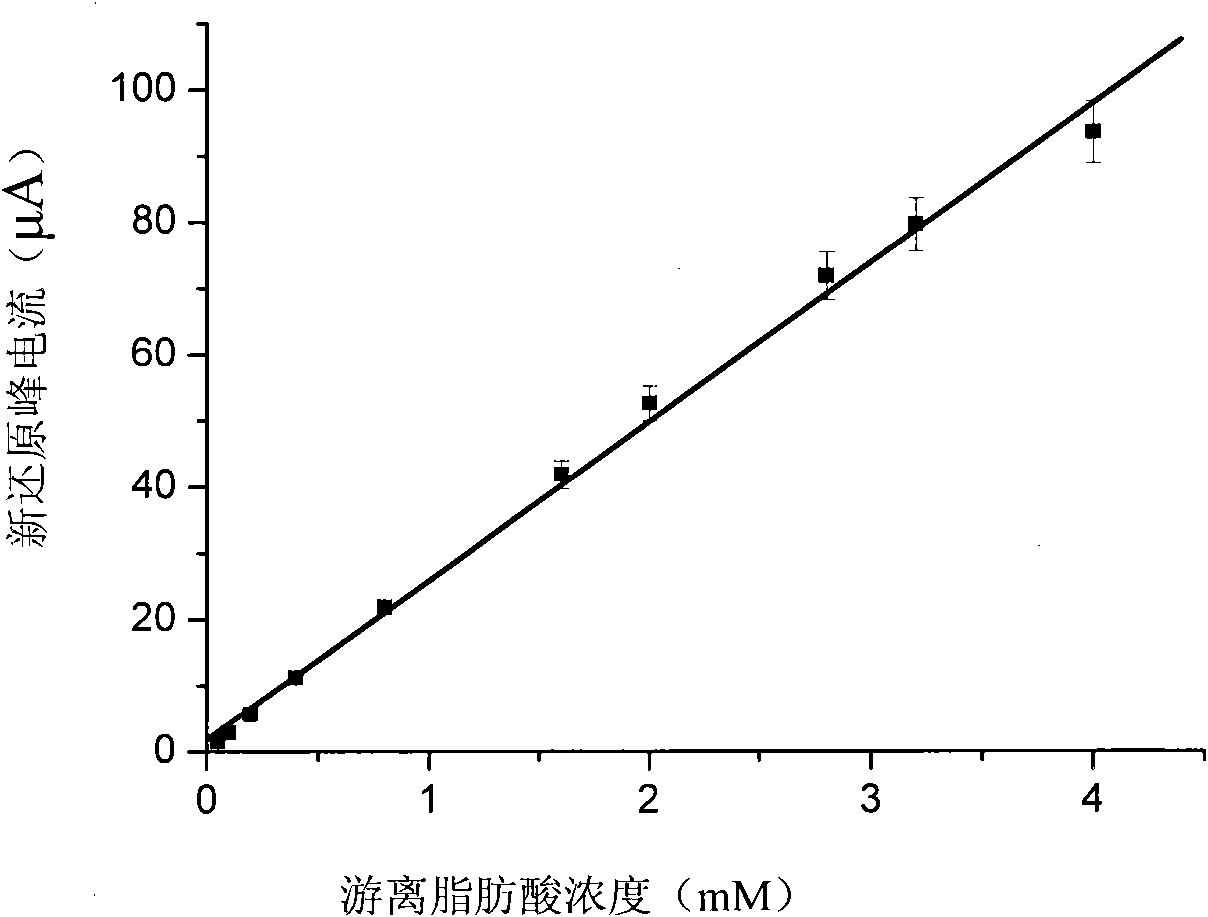
[0047] Prepare 15 modified electrodes under the same electropolymerization conditions, record their linear voltammograms under the same electrolyte solution and the same electrical parameter conditions, do three parallel experiments for each electrode and sample, and take the average value as The measurement result of the current response of each modified electrode, and then calculate the average value of 15 modified electrodes, the result is: 2.1×10 -3 The average value of the reduction peak current of M linoleic acid was 52.6±1.8 μA (n=15, RSD=3.42%).
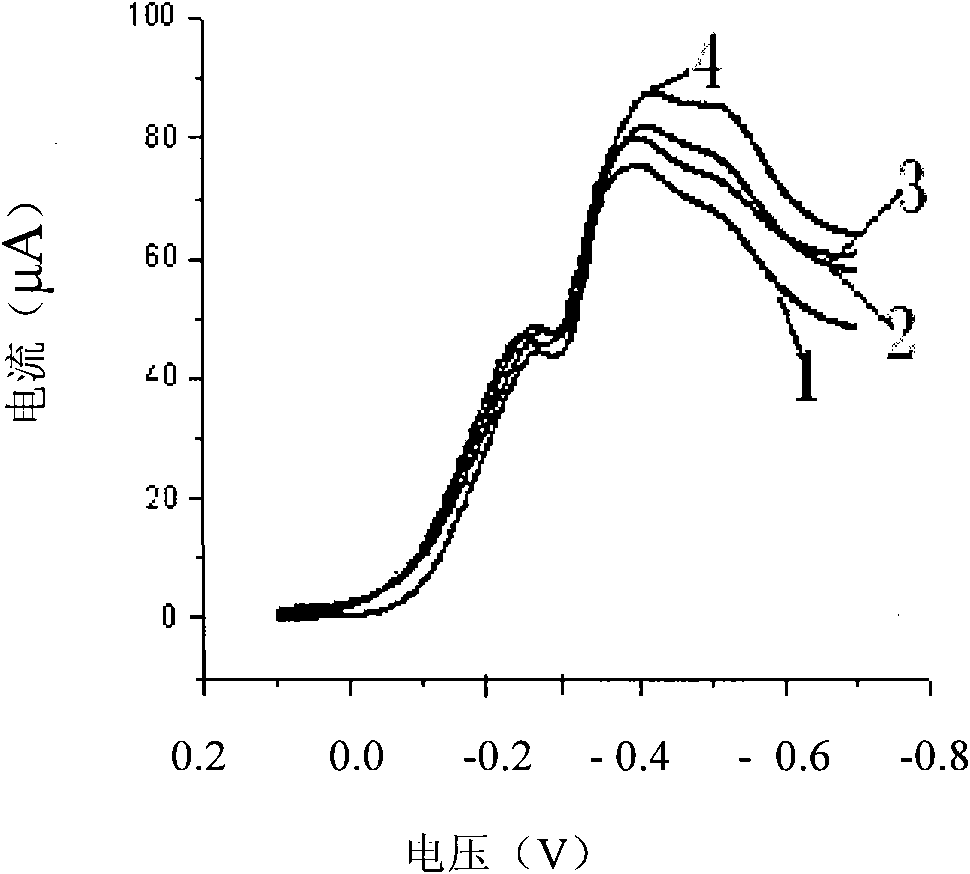
[0048] b. Stability evaluation
[0049] The reduction peak currents of the newly prepared modified electrode and the same modified electrode stored under dry and light-proof conditions for one month we...
Embodiment 3
[0051] This example is a comparative experiment done in order to illustrate that the polypyrrole-doped tetrabutyl perchlorate ammonium modified electrode has better detection performance than the bare electrode.
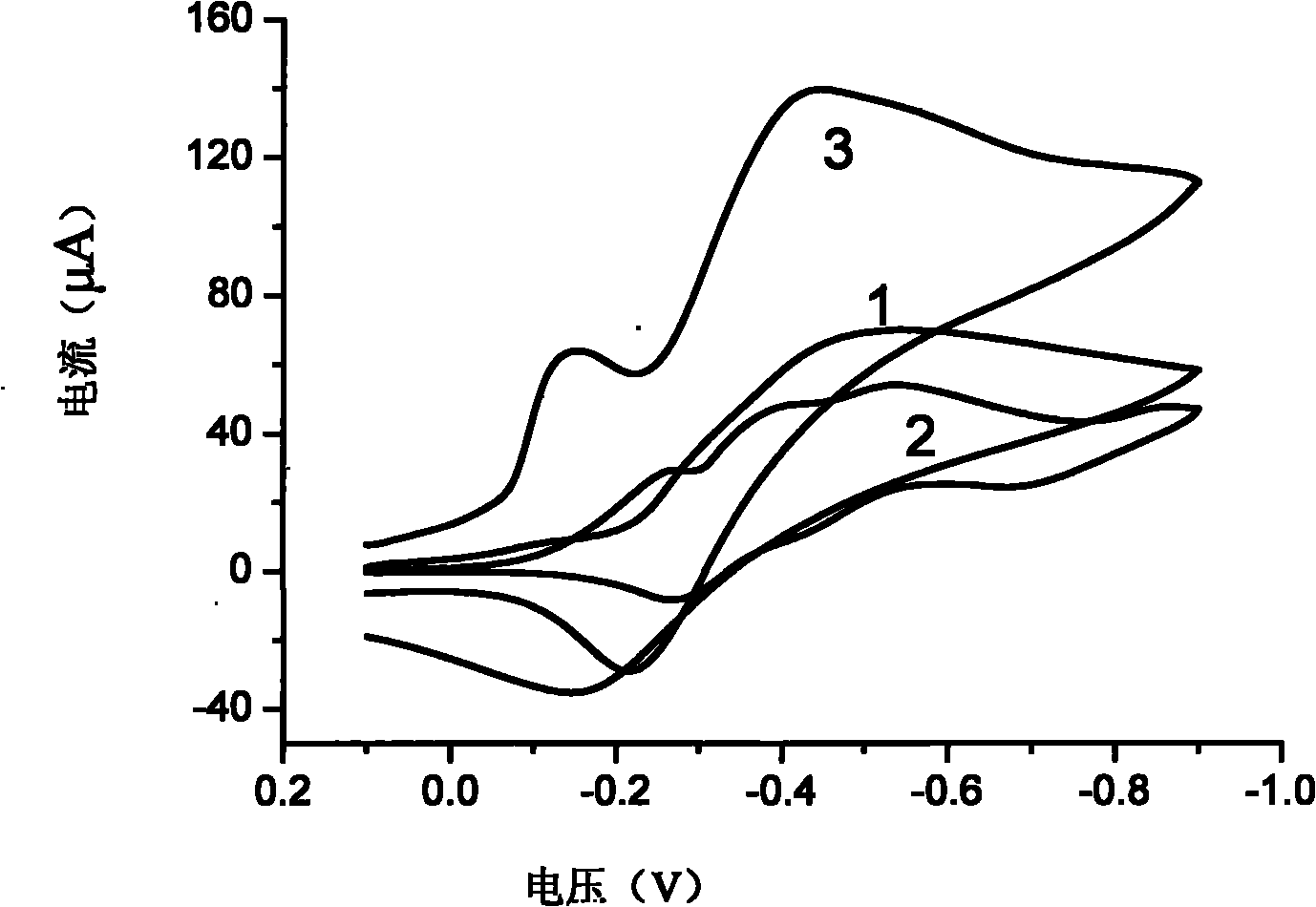
[0052] The specific method is to carry out cyclic voltammetry scanning with two kinds of electrodes respectively in the standard linoleic acid solution of the same concentration, observe their reduction current responses, and find that on the modified electrode, the peak current of linoleic acid reduction increases significantly (about 1.5 ~2 times), the reduction peak potential shifted about 200mV to the positive direction, indicating that the modified electrode has a significant catalytic effect on the electrochemical reduction of linoleic acid. The cyclic voltammograms of the modified electrode obtained by this method and the bare electrode when detecting the same concentration of linoleic acid are as follows: figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com