Method for preparing long chain branching isotactic polypropylene
A technology of isotactic polypropylene and long-chain branching, which is applied in the field of tactic polypropylene and can solve the problems of reduced polymerization activity and low propylene intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Embodiment 1, preparation long chain branched isotactic polypropylene
[0118] In a 500ml stainless steel kettle equipped with a mechanical stirrer, replace with nitrogen and propylene respectively, then add 150ml of toluene and 0.71ml of toluene solution (Al / Zr = 1000), control the temperature at 50°C, add 1 μmol catalyst rac-CH 2 (3-t-Bu-1-Ind) 2 ZrCl 2 (Organometallics 2000, 19, 420-429) (catalyst 1), add 0.71ml concentration of 1.4mol / l MAO toluene solution (Al / Zr=1000) and 1μmol catalyst rac-Me after reacting at 0.1MPa pressure for 10min 2 Si(2-Me-4-Ph-Ind) 2 ZrCl 2 (Organometallics 1994,13,954-963) (catalyst 2), react 20min under 0.1MPa pressure, be that the ethanol solution of 5% (volume ratio) hydrochloric acid terminates reaction with concentration at last, obtain long-chain branched polymer through washing, drying. propylene.
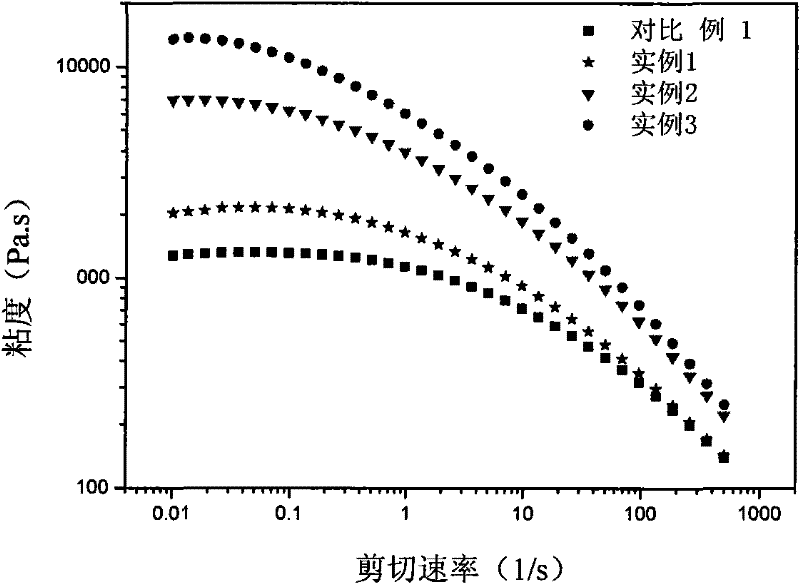
[0119] The rheological curve of the prepared long-chain branched isotactic polypropylene (see figure 1 ). The melting point of...
Embodiment 2
[0120] Embodiment 2, preparation long chain branched isotactic polypropylene
[0121] Replace with nitrogen and propylene respectively in a 500ml stainless steel kettle equipped with a mechanical stirrer, then add 150ml of toluene and 0.36ml of toluene solution (Al / Zr = 1.4mol / l MAO through metal sodium reflux dehydration 1000), control the temperature at 50°C, add 0.5μmol catalyst rac-CH 2 (3-t-Bu-1-Ind) 2 ZrCl 2 (Catalyst 1), add 0.71ml concentration to be the MAO toluene solution (Al / Zr=1000) of 1.4mol / l after reacting 10min under 0.1MPa pressure and 1μmol catalyst rac-Me 2 Si(2-Me-4-Ph-Ind) 2 ZrCl 2 (Catalyst 2), reacted under 0.1MPa pressure for 20min, and finally terminated the reaction with ethanol solution of 5% (volume ratio) hydrochloric acid, washed and dried to obtain long-chain branched polypropylene.
[0122] The rheological curve of the prepared long-chain branched isotactic polypropylene is shown in figure 1 . The results of the measured melting point an...
Embodiment 3
[0125] Embodiment 3, preparation long chain branched isotactic polypropylene
[0126] A 500ml stainless steel kettle equipped with a mechanical stirrer was replaced with nitrogen and propylene respectively, and then 150ml of toluene and 0.43ml concentration of 1.4mol / l MAO toluene solution (Al / Zr=3000 ), control the temperature at 50℃, add 0.2μmol catalyst rac-CH 2 (3-t-Bu-1-Ind) 2 ZrCl 2 (Catalyst 1), add 3.9ml concentration is 1.4mol / l MAO toluene solution (Al / Zr=3000) and 1.8μmol catalyst rac-Me after reacting 10min under 0.1MPa pressure 2 Si(2-Me-4-Ph-Ind) 2 ZrCl 2 (Catalyst 2), reacted under 0.1MPa pressure for 20min, and finally terminated the reaction with ethanol solution of 5% (volume ratio) hydrochloric acid, washed and dried to obtain long-chain branched polypropylene.
[0127] The rheological curve of the prepared long-chain branched isotactic polypropylene is shown in figure 1 . The results of the measured melting point and intrinsic viscosity of the polyme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com