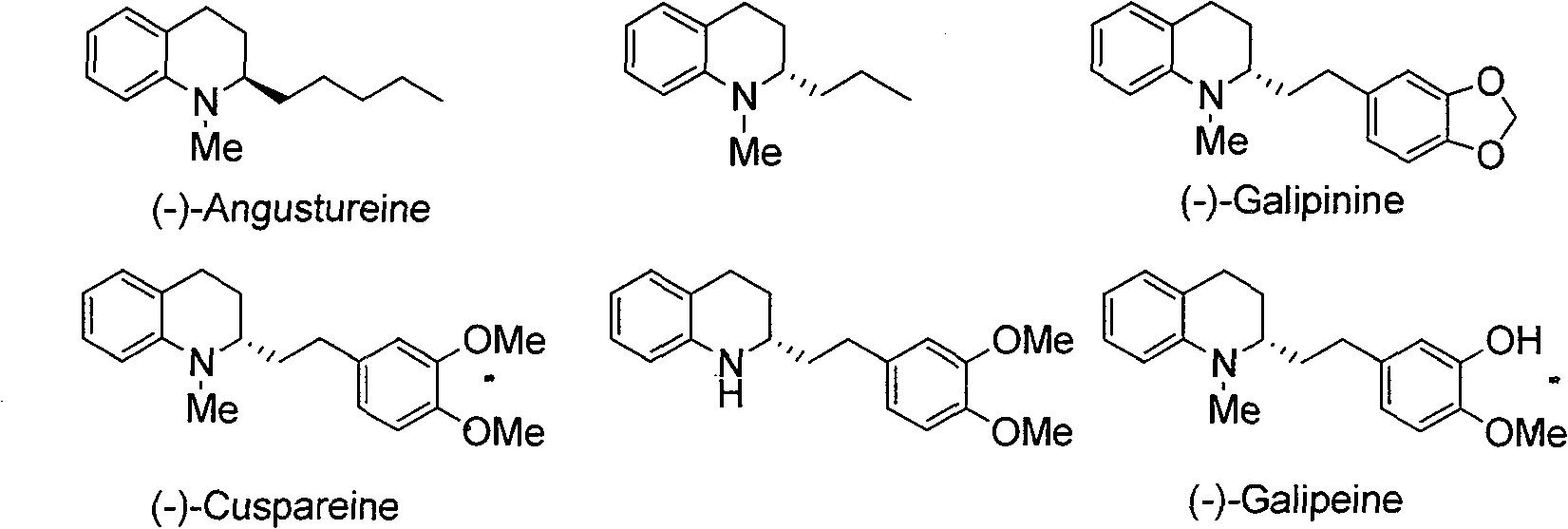
Method for synthesizing derivatives of chiral tetrahydroquinoline by catalyzing asymmetric hydrosilylation with iridium
A derivative, asymmetric technology, used in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve problems such as high pressure, achieve complete reaction, high reactivity and enantioselectivity, To achieve the effect of chiral value-added
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: optimization of conditions
[0027] Add iridium precursor (1.7mg, 0.0025mmol) and chiral ligand (0.0055mmol) into the reaction bottle, add 1ml of tetrahydrofuran after nitrogen replacement, and stir at room temperature for 10 minutes. Then elemental iodine (6.4mg, 0.025mmol) was added and stirred at room temperature for 10 minutes. Afterwards, substrate 1a (37mg, 0.25mmol), water (9mg, 0.5mmol) and triethylsilylhydrogen (174mg, 1.50mmol) were added, and finally 2ml of tetrahydrofuran was added, and the reaction was stirred at room temperature for 24 hours. The enantiomeric excess of the product was determined by chiral liquid chromatography, see Table 1. The reaction formula and ligand structure are as follows:
[0028]
[0029] Table 1. Optimization of conditions for iridium-catalyzed asymmetric hydrosilylation of 2-methylquinoline 1a
[0030] serial number
Embodiment 2
[0031] Example 2: Synthesis of Tetrahydroquinoline Derivatives 2 by Iridium-Catalyzed Asymmetric Hydrosilylation
[0032] Add iridium metal precursor (1.7mg, 0.0025mmol) and chiral ligand (3.4mg, 0.0055mmol) into the reaction bottle, add 1ml of tetrahydrofuran after nitrogen replacement, and stir at room temperature for 10 minutes. Then elemental iodine (6.4mg, 0.025mmol) was added and stirred at room temperature for 10 minutes. Afterwards, add substrate 1 (0.25mmol), water (9mg, 0.5mmol) and triethylsilylhydrogen (174mg, 1.50mmol), and finally add 2 milliliters of tetrahydrofuran, stir at room temperature and detect the reaction by TLC, stop stirring when the reaction ends . The reaction time is 24-72 hours, and the enantiomeric excess of the product is determined by chiral liquid chromatography, as shown in Table 2, and the reaction formula is as follows:
[0033]
[0034] Table 2. Iridium-catalyzed asymmetric hydrosilylation synthesis of various chiral tetrahydroquinol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com