Multi-portion intra-oral dosage form with organoleptic properties
An organoleptic, multi-part technology, applied in the direction of organic active ingredients, medical preparations containing active ingredients, inhalers, etc., which can solve the problems of introduction compliance and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
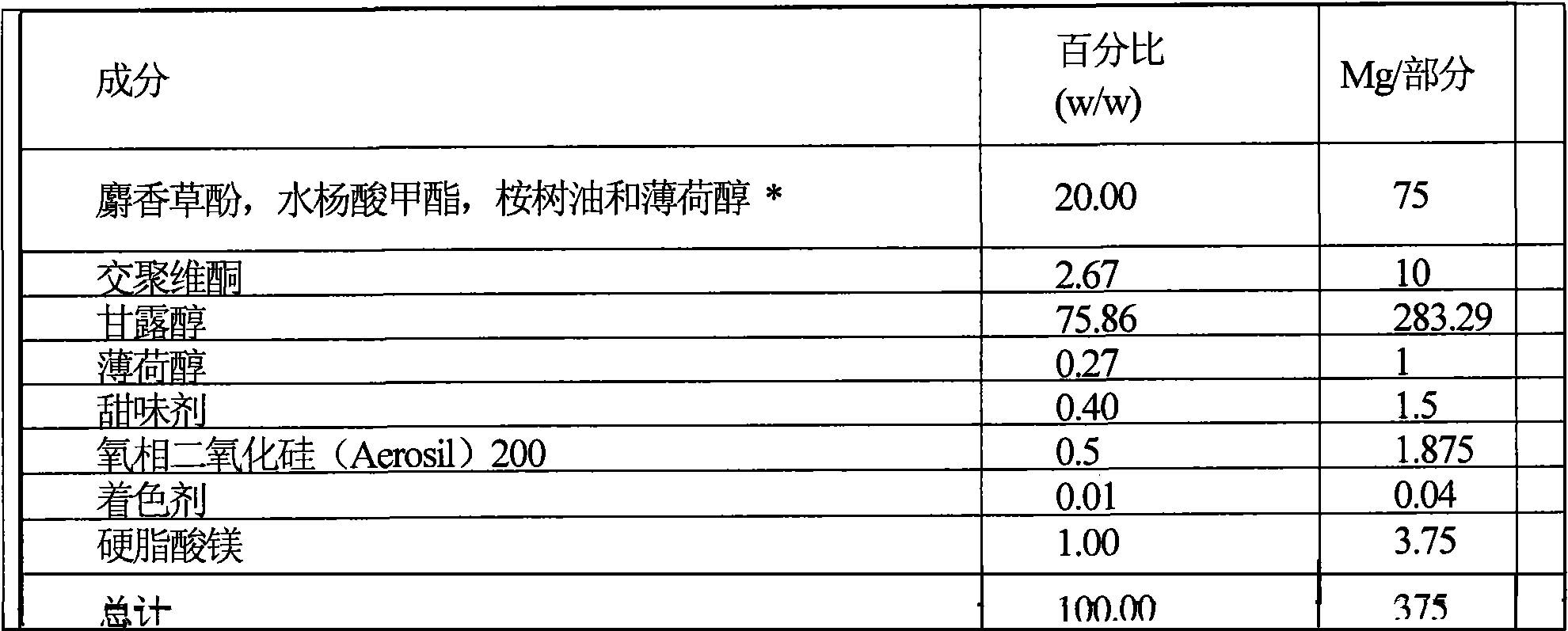
[0142] Preparation of two-part tablets, each part of which has different flavor, cooling intensity and hardness. The fast dissolving part contains 0.5mg nicotine (NRC) and has a menthol flavor, and the slow dissolving part contains 1.5mg nicotine (NRC) and has a lemon fragrance. Therefore different fragrances are associated with different parts of the pharmaceutically active agent, and the pharmaceutically active agent is released at different rates. The dissolution end of the rapidly disintegrating part and the lemon scent suggest that approximately 25% of the pharmaceutically active agent has been released.
[0143] Preparation
[0144] The ingredients listed in Table A1 and Table A2 are each sieved according to a known method in the prior art, such as a double cone mixer, and then mixed. Then the two parts of the mixture are compressed into tablets by a direct compression device. Powder compression can be implemented, for example, using a double-sided rotary tablet press with...
Embodiment 2
[0153] The preparation of two-part tablets, each part has different texture and flavor, one part has rough geometric pattern or shape or form, and the other part has smooth surface. The initial mint scent provides an organoleptic sensation that prompts the patient to start the release of the pharmaceutically active agent, while the cinnamon scent and the rough surface texture of the corresponding part are indicative of a buffering agent that promotes the absorption of the pharmaceutically active agent. Tablet preparation also facilitates the inclusion of drugs with potential compatibility issues.
[0154] Preparation
[0155] The same method as in Example 1 was used, but the upper punch had a rough geometric pattern.
[0156] Table B1: Composition of parts with rough geometric patterns
[0157]
[0158] Table B2: Composition of parts with smooth geometric patterns
[0159]
[0160] * Equivalent to 2.0mg dose of nicotine base
Embodiment 3
[0162] Two-part tablets are prepared, each part of which has a different texture, one part is harder and has a rough geometric pattern, and the other part is softer and has a smooth surface.
[0163] Preparation
[0164] The same method and the same formula as in Example 1 were used, but the upper punch used had a rough geometric pattern. Further, no fragrance is added. Therefore, the difference in flakeability / fragmentation between different parts becomes more significant than in Example 1. The "smell of nicotine" in itself is used as a conceptual aid to the patient: the pharmaceutically active agent has begun to be released, and the rapid dissolution of flakes / fragments and smooth surface parts will serve as an indication to the patient: about 25% The pharmaceutically active agent has been released from the dosage form.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle diameter | aaaaa | aaaaa |
| Average particle diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com