Method for semi-synthesizing paclitaxel and docetaxel
A docetaxel, semi-synthetic technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of low yield of final products and high production cost, and achieve the effects of easy realization, high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
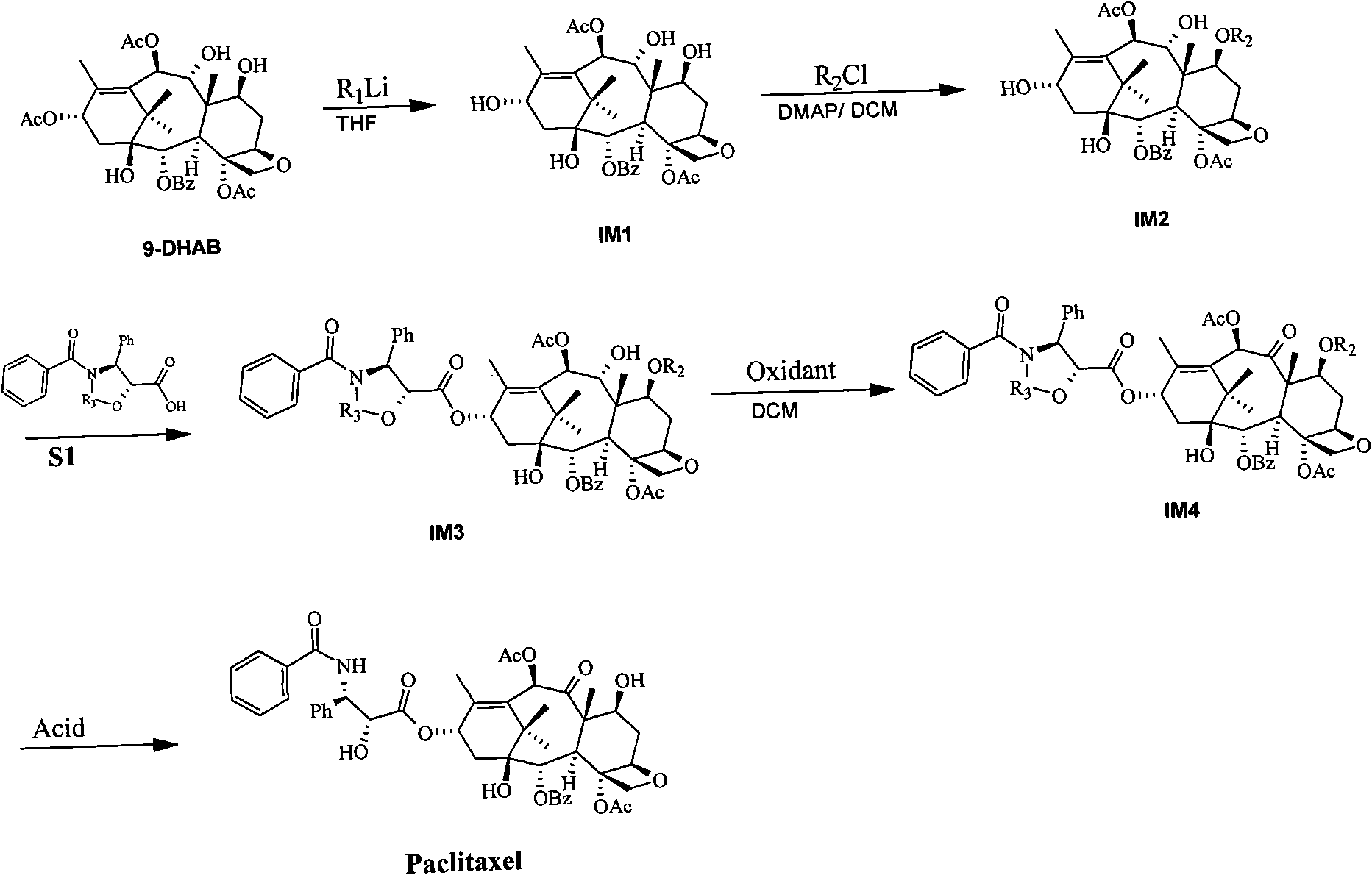
[0050] Step 1, the preparation of 9-dihydrobaccatin III (compound IM1)
[0051] 100g of 13-acetyl-9-dihydrobaccatin III (9-DHAB) was dissolved in 2L of tetrahydrofuran (THF), the temperature of the reaction system was lowered to -70°C, and then 600 ml of 1.6mol / l methyllithium was added dropwise into the reaction system and maintain the temperature of the reaction system at -70°C for 3 hours. Sampling was carried out to monitor the reaction, and until the raw materials were completely consumed, ammonium chloride solution was added to the reaction system to stop the reaction. The organic layer was washed with brine, dried and concentrated. The concentrate was recrystallized with 200 ml of dichloromethane, and filtered to obtain 74 g of 9-dihydrobaccatin III (compound IM1), with a yield of 80%.
[0052] Step 2, Preparation of 7-methyldiphenylsilyl-9-dihydrobaccatin III (Compound IM2)
[0053] 74g of compound IM1 and 35.3g of DMAP (4-dimethylaminopyridine) were dissolved in 1L o...
Embodiment 2
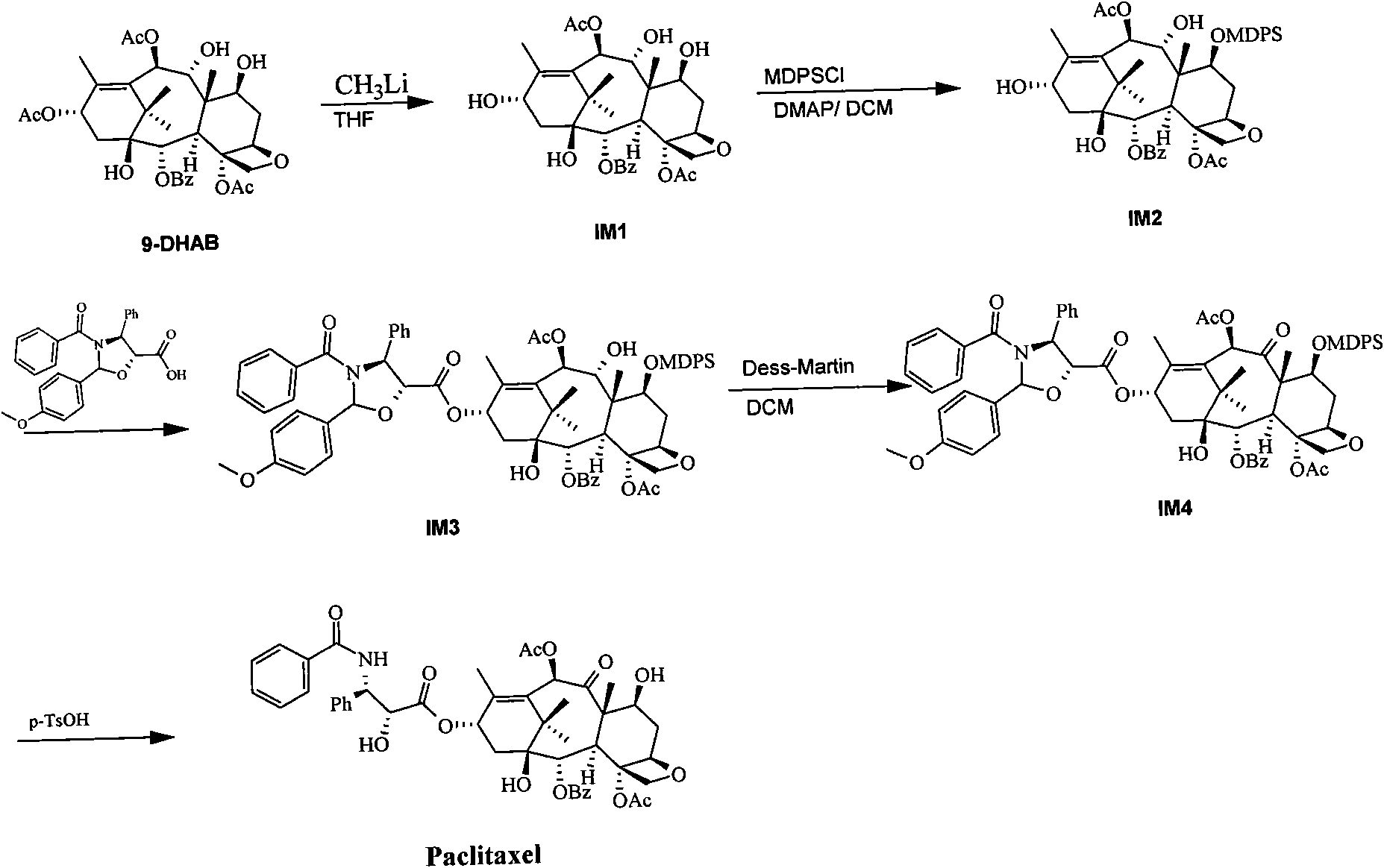
[0061] Step 1, the preparation of 9-dihydrobaccatin III (compound IM1)
[0062] Dissolve 100g of 13-acetyl-9-dihydrobaccatin III (9-DHAB) in 2L of tetrahydrofuran, lower the temperature of the reaction system to -70°C, and then add 600ml of 1.6mol / l methyllithium dropwise into the reaction system , the temperature of the reaction system was maintained at -70°C for 3 hours. Sampling was carried out to monitor the reaction, and until the raw materials were completely consumed, ammonium chloride solution was added to the reaction system to stop the reaction. The organic layer was washed with brine, dried and concentrated. The concentrated solution was recrystallized with 200 ml of dichloromethane, and 74 g of compound IM1 was obtained after filtration, with a yield of 80%.
[0063] Step 2, Preparation of 7-methyldiphenylsilyl-9-dihydrobaccatin III (compound IM2)
[0064] 74g of compound IM1 and 35.3g of DMAP were dissolved in 1L of dichloromethane, the temperature of the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com