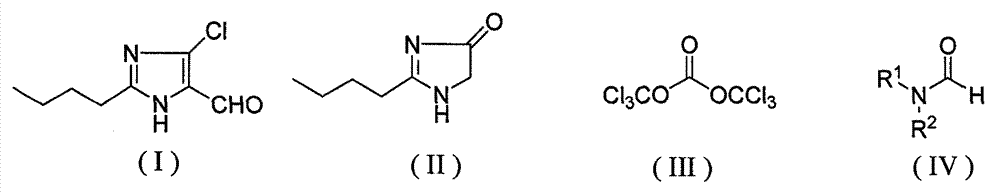
Method for synthesizing 2-normal-butyl-4-chloro-5-formylimidazole
A technology of formyl imidazole and n-butyl is applied in the field of synthesis of losartan intermediate 2-n-butyl-4-chloro-5-formyl imidazole, which can solve the problems of difficult separation and purification of products, high cost of waste treatment, Harsh reaction conditions and other problems, to achieve the effect of low cost of waste treatment, easy separation and purification, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 250mL four-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add 14g (0.1mol) of imidazolone (II), 50mL of toluene, stir to dissolve, add 21.9g of N,N-methylformamide (DMF) (0.3mol), bis(trichloromethyl)carbonate 29.7g (0.1mol), stirred for 30 minutes, then heated to 100°C for 4 hours. After the reaction is complete, add 20 mL of water, stir and hydrolyze for 10 minutes, separate the water layer after standing, extract the water layer with 20 mL of toluene, combine the toluene layers and dry with anhydrous sodium sulfate, evaporate the solvent, add ethyl acetate / petroleum ether ( Volume ratio 1:1) 80mL, after recrystallization, 14.0g light yellow solid was obtained, melting point 93-94°C, yield 75%.
Embodiment 2
[0025] In a 500mL four-necked flask equipped with a thermometer, a reflux condenser and mechanical stirring, add 28g (0.2mol) of imidazolone (II), 100mL of xylene, stir to dissolve, add N, N-diethylformamide 60.6g ( 0.6mol), bis(trichloromethyl)carbonate 59.4g (0.2mol), stirred for 30 minutes, then heated to 100°C for 4 hours. After the reaction is complete, add 30 mL of water, stir and hydrolyze for 10 minutes, separate the water layer after standing still, extract the water layer with 50 mL of xylene, combine the xylene layers and dry with anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and add ethyl acetate / petroleum ether (volume ratio 1:1) 120mL, after recrystallization, 26.0g of light yellow solid was obtained, the melting point was 92-94°C, and the yield was 70%.
Embodiment 3
[0027] In a 250mL four-neck flask equipped with a thermometer, reflux condenser and mechanical stirring, add imidazolone (II) 14g (0.1mol), toluene 50mL, stir to dissolve, add N-phenyl-N-methylformamide 40.5g (0.3mol), bis(trichloromethyl)carbonate 29.7g (0.1mol), stirred for 30 minutes, then heated to 100°C for 4 hours. After the reaction is complete, add 20 mL of water, stir and hydrolyze for 10 minutes, separate the water layer after standing, extract the water layer with 20 mL of toluene, combine the toluene layers and dry with anhydrous sodium sulfate, evaporate the solvent, add ethyl acetate / petroleum ether ( Volume ratio 1:1) 80mL, 11.2g of light yellow solid was obtained after recrystallization, melting point 90-93°C, yield 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com